Next-Generation Virtual Experience
In this highly interactive session, attendees will have the opportunity to explore how the thoughtful integration of Quality Risk Management (QRM) and Knowledge Management (KM) can lead to more informed decision making across the Pharmaceutical Quality System (PQS) during technology transfer and commercial manufacturing. The discussion will be led by regulatory science researchers from TU Dublin and industry experts.
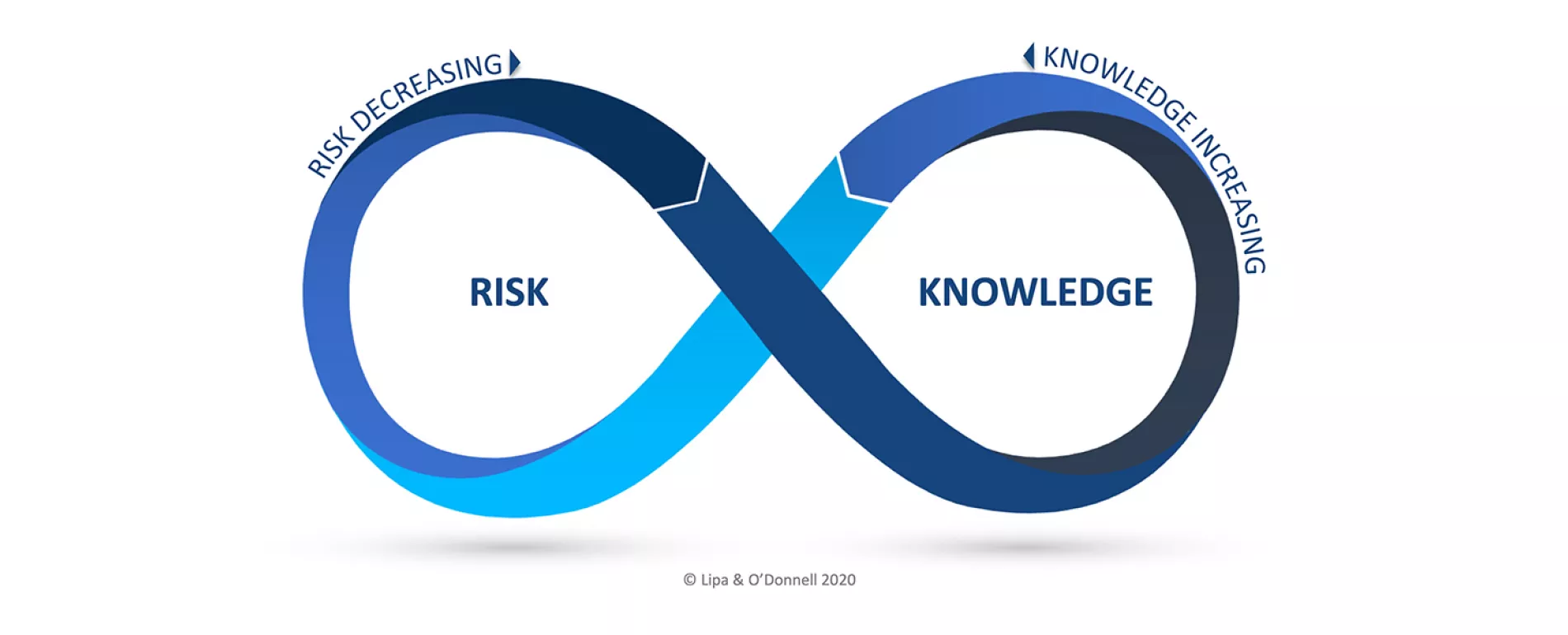
A novel framework to intentionally unite QRM and KM, the Risk-Knowledge Infinity Cycle (RKI Cycle), was first introduced in May 2021 through the ISPE Good Practice Guide on Knowledge Management in the Pharmaceutical Industry and an ISPE Webinar, Exploring the RKI Cycle Across the Product Lifecycle. The RKI Cycle will be presented in brief to orient the attendees, followed by a presentation on risk-based decision making. These presentations will be followed by breakout sessions to engage with the experts and fellow participants on how they view the potential opportunity and impact of the RKI Cycle, as well as potential implementation challenges.
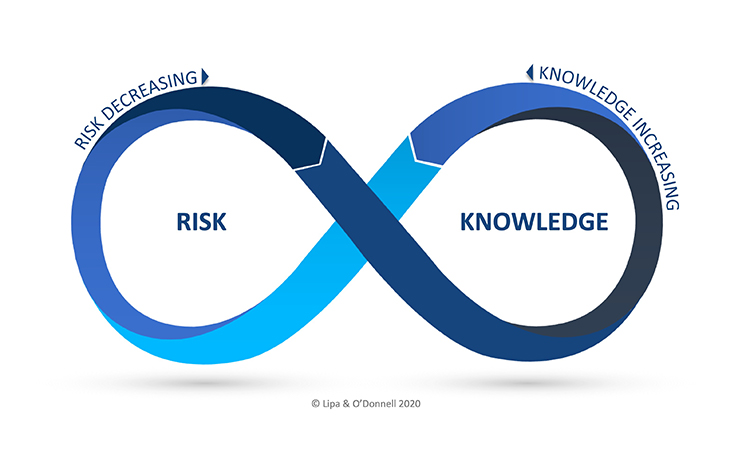
Attendees will have the opportunity to engage in big picture thinking regarding the underlying intent of ICH Q10 during technology transfer and commercial manufacturing for organizations to make informed decisions based on science and evidence. The attendees will furthermore get the opportunity to participate, reflect and engage in two-way dialog on what types of scenarios and challenges stand to benefit most from a framework such as the RKI Cycle. In the spirit of the Expert Xchange, the participants will benefit from in hearing from cross-functional experts on the RKI Cycle, KM, and QRM, as well as fellow practitioners.
The outputs of this session will be captured in a paper, anticipated to be published in ISPE Pharmaceutical Engineering in 2022.
Hurry – space is limited for this highly interactive session!
Don’t miss out on this next-generation virtual experience – ISPE Expert Xchange!
*ISPE Membership is required for these registration rates.
Bring your team to an ISPE Expert Xchange
Group discount applies to Member/Nonmember rate only. To qualify, all registrant information must be submitted at the same time. Registrations that arrive later will NOT be eligible for the group discount. Cannot be combined with other offers. To register as a group, please contact ISPE by telephone, +1-813-960-2105 or by email, ask@ispe.org.