Features
Dr. Gabriella Dahlgren, PhD

Senior Manager, Strategy Deployment and Excellence, PQM
Related Articles
Features
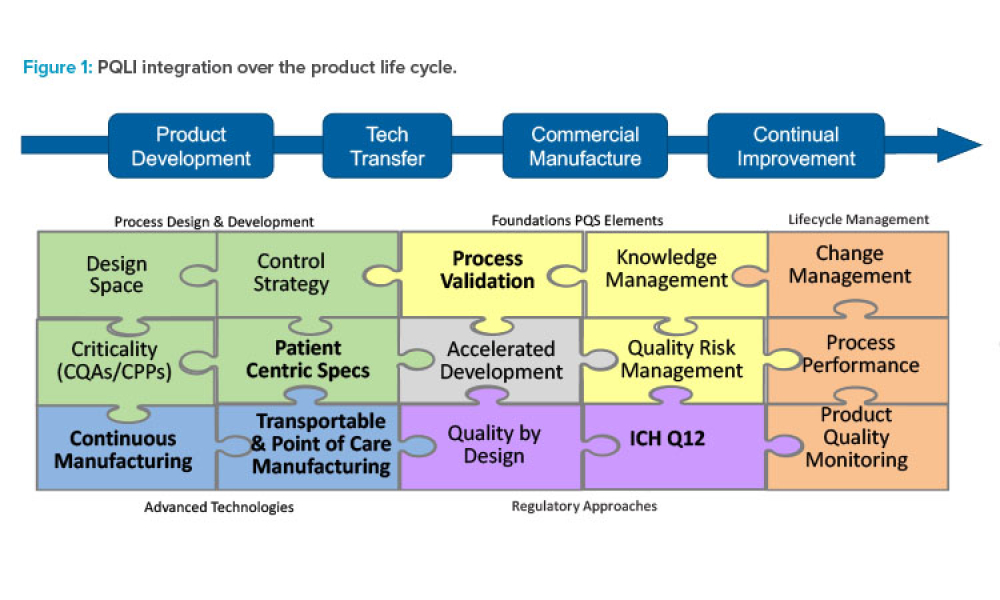
A unique aspect of the pharmaceutical industry is the pairing of innovation and regulation. For nearly two decades, ISPE’s Product Quality Lifecycle Initiative (PQLI®) has worked at the nexus of...
Technical
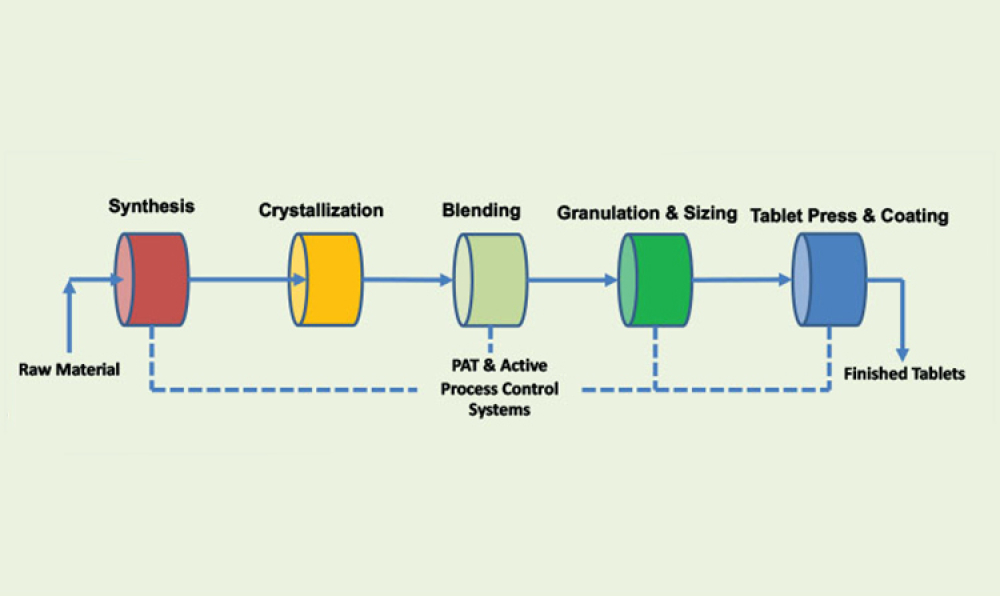
Process analytical technology (PAT) is perceived as the main enabler for a robust control strategy with continuous manufacturing (CM) because process analytical technology can aid in implementing continuous manufacturing throughout the entire life cycle. This article discusses quality and regulatory hurdles in the life cycle of a process analytical technology application—including model...