Technical
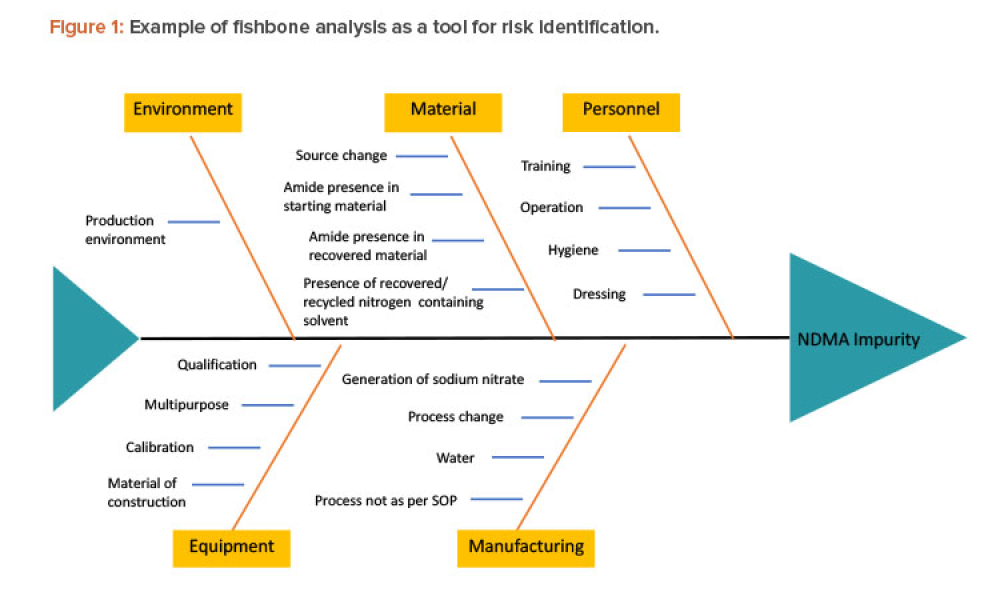
Recently, recalls of angiotensin receptor antagonists, particularly valsartan, and warning alerts about N-nitrosodimethylamine (NDMA) impurities in drug substances such as ranitidine and metformin have demonstrated the urgent need for manufacturers and regulators to control impurities throughout the product life cycle to ensure patient safety.