ISPE starts 2022 with a bang, with the 31st Annual Face-to-Face 2022 ISPE Aseptic Conference, March 14-15, offering attendees several unique perspectives and case studies across various...
Competitive advantage and sustained business results depend on leaders building and maintaining a strong culture. This has been reinforced by the increased challenges in attracting and retaining talent. The FDA is also placing significant focus on Quality Management Maturity. But how do leaders know if they are on track? Assessing leadership systems is often overlooked or much more difficult...
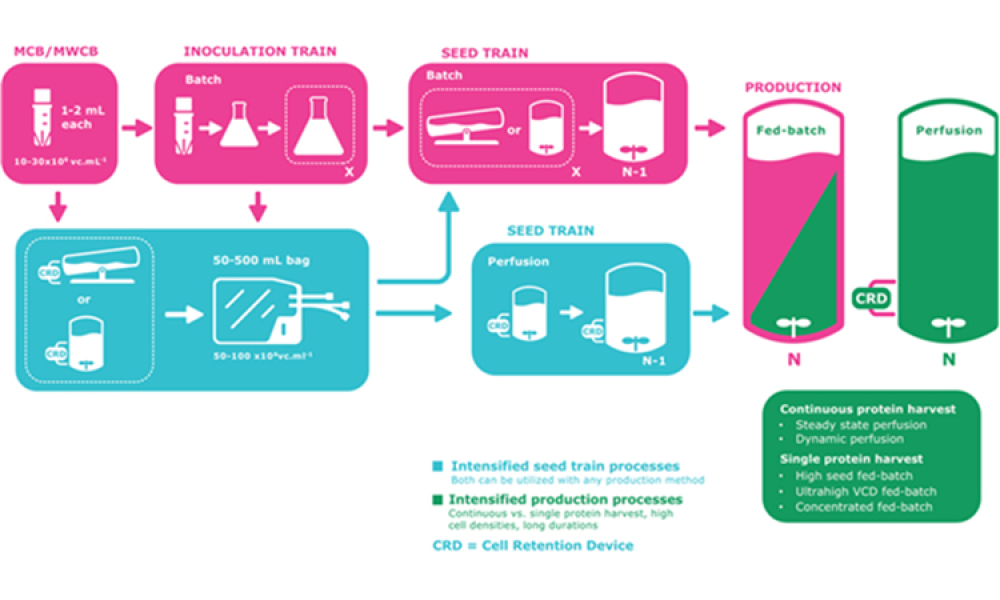
Vaccine Manufacturing
The industry is experiencing an acceleration in the development and approval of novel prophylactic and therapeutic vaccine modalities. Concurrently, many biopharmaceuticals are experiencing the benefits of manufacturing process intensification (PI). Describing such advances for cell-culture based processes is challenging in the field of vaccinology because of...
It is a great honour and pleasure for me as Chair of the ISPE Board of Directors and Chair of the 2022 ISPE Aseptic Conference Program Committee to give you an overview of the upcoming conference including information about the knowledge that will be shared, a look at some of the speakers, and details about a few of the networking opportunities.