As a young Science graduate, I couldn’t think of anything worse than being stuck at the same desk, day in / day out, in a stuffy office, doing the same thing day after day, just to earn a living. There had to be more to life, right? I wanted to travel the world, see new places, not be defined by what I do or how I look, and most definitely not be pigeonholed due to my career choice or hair...
The main change can be seen on the imports of IMPs from EU/EEA to Great Britain starting 01 January 2022. Sponsors of United Kingdom Clinical trials will need to appoint a United Kingdom Manufacture & Importation Authorization (MIA) (IMP) holder who will be responsible for implementing an oversight process to confirm each batch has been certified by a Qualified Person before its release to...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from April 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
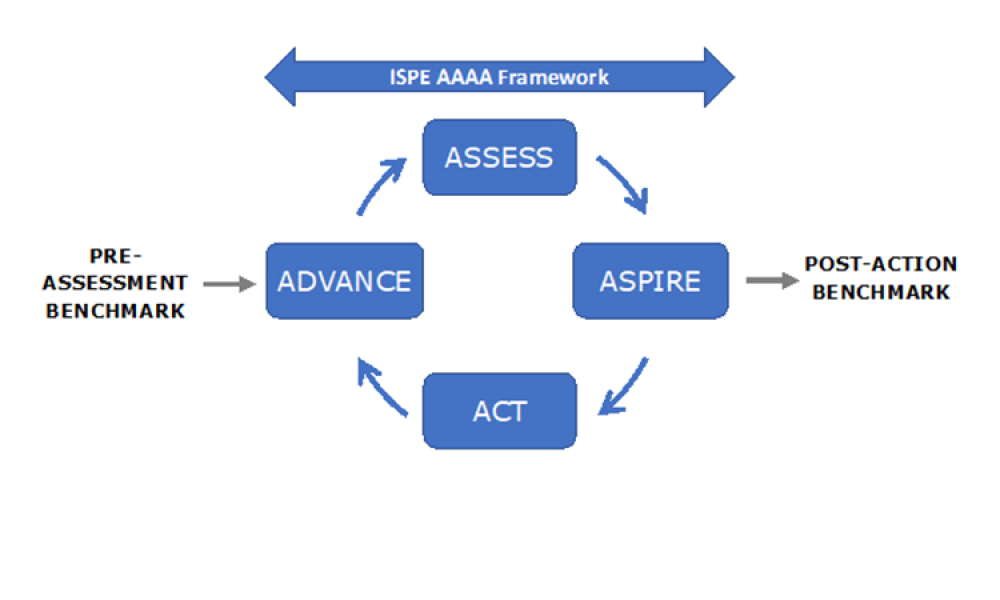
While Pharmaceutical development and manufacturing have focused for years on robust quality management systems (QMS), the application of quality risk management (QRM) applying digital tools and advances are critical to future success. Coupling controls, artificial intelligence, and associated platforms with basic QMS and QRM affords a great opportunity for the pharmaceutical industry.
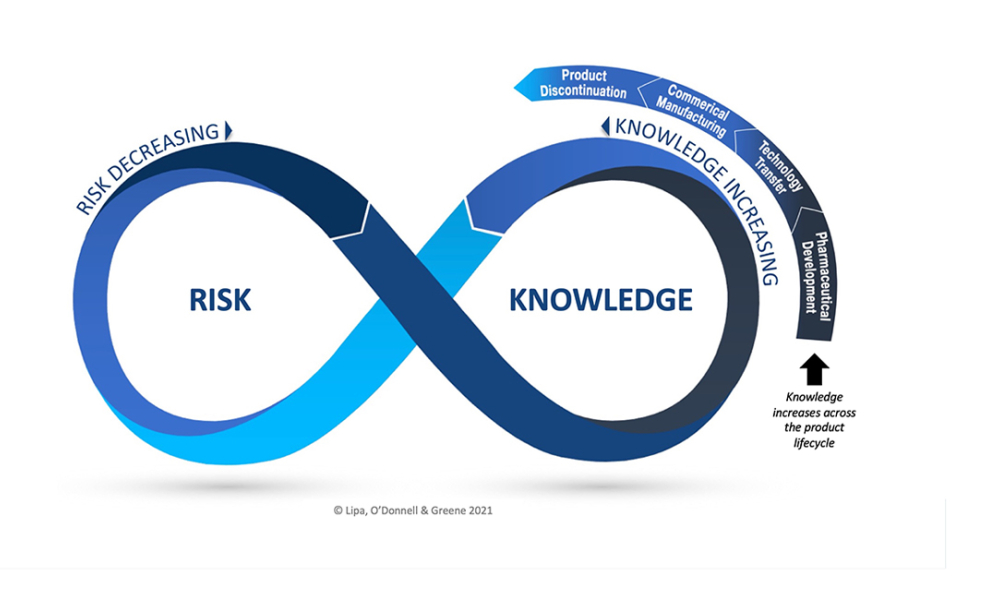
Case studies demonstrating the link between knowledge and risk in technology transfer and commercial manufacture
Economic Operators Registration and Identification (EORI) Number
Customs procedures also imply having an Importer of Record liable at destination as well as having each party, exporter, and importer, registered, and identified with its own EORI (from the Country it is based at). An XI EORI number is required to move goods between NI and non-EU Countries (which comprise Great...