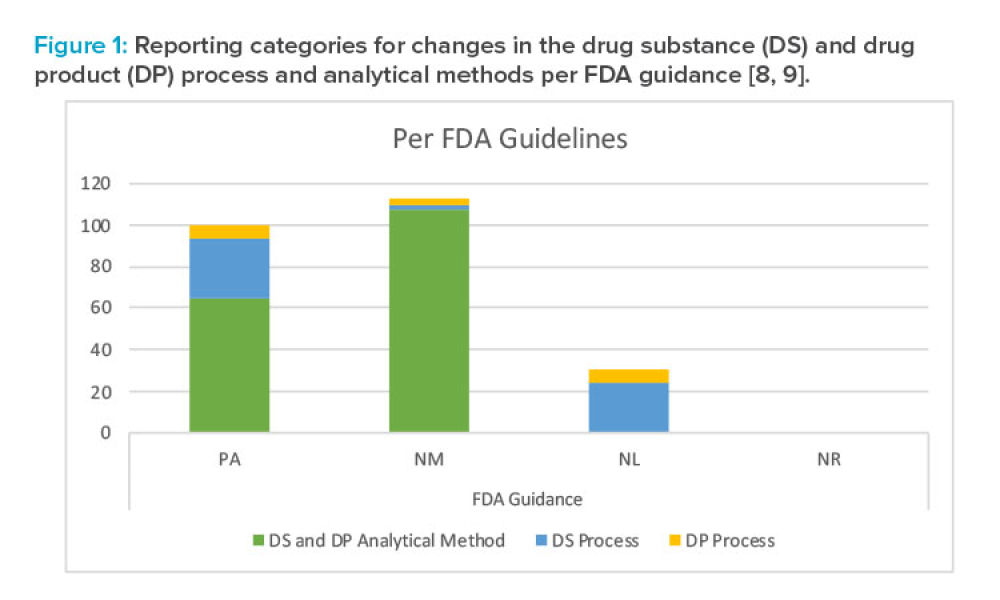
Management of global postapproval chemistry, manufacturing, and controls (CMC) changes is a growing challenge for industry with many issues. ICH Q12 (Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management) is a transformative document shaping global regulatory postapproval submissions that will help alleviate some of these issues.
Features