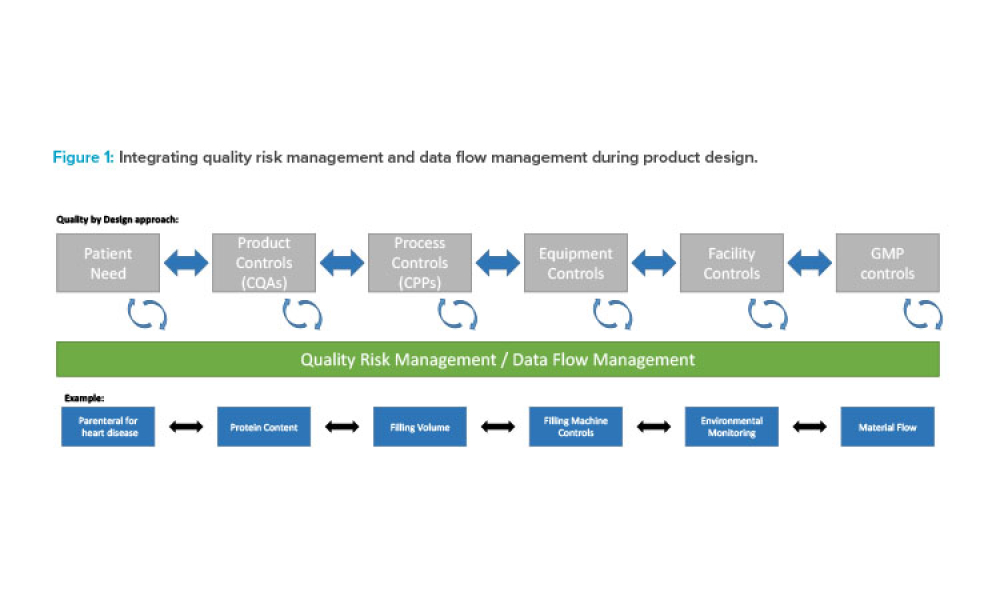
Features
Michelle Vuolo

Tulip Interfaces
Head of Quality
Michelle currently leads Quality at Tulip Interfaces, Inc, who develops and sells a frontline operations platform where manufacturers of many industries can build digital content to manage their operations. Before joining Tulip Michelle spent over 24 years in the biopharmaceutical and medical device industries in many different roles ranging from QC laboratory, engineering technical support, Quality Assurance management and Computerized Systems Compliance. Michelle has a strong understanding of the needs of the life sciences industry and is now motivated and driven to evolve stagnant ways of meeting compliance requirements, especially as it relates to the 4.0 world. "My main mission is to stay current in our ever changing world. Staying current means to be outwardly active, remain open to evolution, and seek efficient, effective means to meet needs in new ways.”