Digitization, Technologies, and More at 2019 ISPE Europe Annual Conference

More than 800 attendees met in Dublin, Ireland, on 1–4 April for the 2019 ISPE Europe Annual Conference—a new record attendance for this conference! Participants at ISPE’s sixth Europe conference learned about how manufacturers, key suppliers, functional peer groups, and regulators expect the pharmaceutical industry to be affected by digitization, new products, changing portfolios, disruptive technologies, and other factors.
The speakers and others at the conference demonstrated an enthusiastic commitment to finding answers to the industry’s challenges. Impressive case studies represented a range of perspectives. Presenters emphasized that pharmaceutical products deserve special attention because no other products affect health and safety as much as pharmaceutical products. Increasingly, health and disease management is managed by the patients themselves, so patients are partners with other stakeholders in the pharmaceutical network, and they can be empowered by new technologies, such as apps, as well as direct communication.
Innovation, at all levels and for all stakeholders, is key to success. The conference attendees made it clear that taking an active role in changes is better than simply following trends.
Executive Forum
A highlight of the conference’s first day was the Executive Forum, in which speakers offered the audience a high-level perspective, including discussions of major developments in the political boundaries in Europe as well as important regulatory developments related to product life-cycle management.
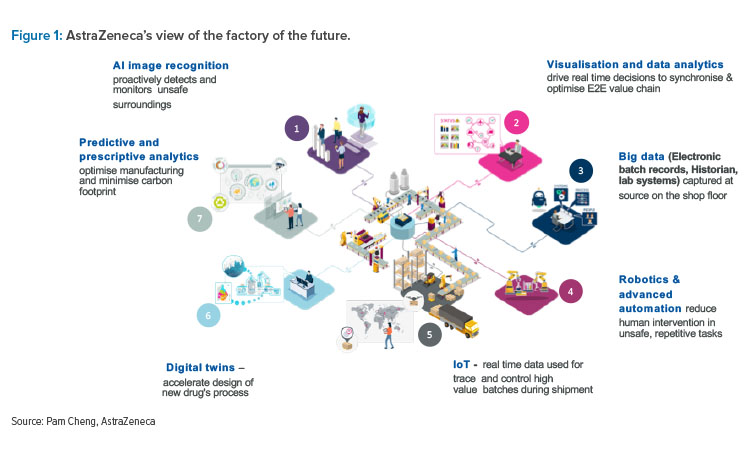
Pam Cheng, Executive Vice President of Global Operations and IT at AstraZeneca, stressed that digital technology will change everything in pharma. Driven by rising costs and new demands for innovation in health systems worldwide, stakeholders will increase their scrutiny of value. Therefore, the industry needs to invest in new digital solutions to solve research, development, and access challenges and drive differentiation in the market. Cheng noted that breakthroughs in digital technology are redefining society and the practice of medicine and explained that AstraZeneca is putting greater emphasis on advancing ever-more innovative science, being more patient-centered, and doing more with digital technology and data. Among the company's areas of technological focus are three-dimensional printing, virtual reality, voice-directed technology, artificial intelligence (AI), digital twins, connected drones, machine learning, and the internet of things (IoT). Figure 1 illustrates the company’s view of the factory of the future.
Olivier Loeillot, General Manager of Bioprocess at GE Healthcare, presented on biopharma of tomorrow and rethinking efficiency. He addressed the challenges involved in bioprocessing, emphasizing that considerable investment is required while a molecule’s likelihood of success is still low. Only 5% of oncology and 12% of nononcology drugs successfully make it to market.
He further noted that it takes an average of 12 years for a drug to go from concept to market. Small batch sizes are becoming more common, driven by biosimilar production, personalized medicine, and higher monoclonal antibody titers.
In production, it is expected that there will be no downtime and quality will meet the highest standards. For example, in cell therapy, there is zero tolerance for production failures. Therefore, real-time preventive alerts and a process-monitoring dashboard are essential for 100% assurance that every patient batch is correct.
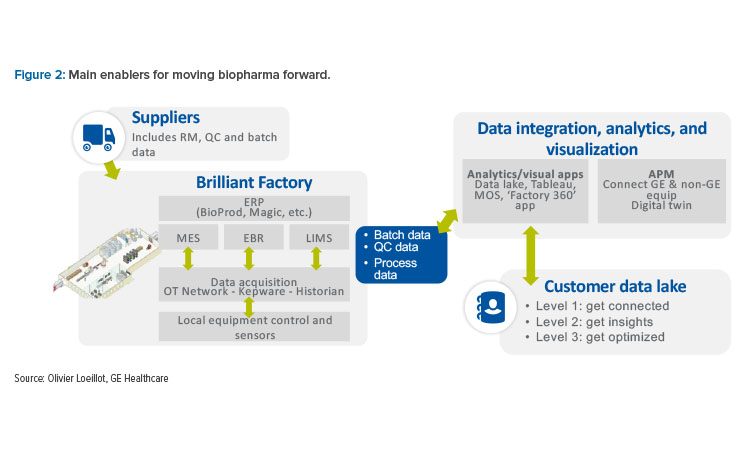
In procurement, manufacturers need to address errors and events rapidly and remotely to isolate the root causes of failure. Loeillot stated that the main enabler for moving biopharma forward is interconnected digital platforms to integrate, analyze, and visualize data for suppliers, factories, and customers (Figure 2).
Chris Chen, Chief Executive Officer of WuXi Biologics, reported that his company will have a state-of-the-art biologics production facility established in Ireland within the next two years. WuXi Biologics’ strategy is to use disposable bioreactors as disruptive technology and to consider the merits of scale-out (numbering up) versus the scale-up (sizing up) principles in production. Furthermore, drivers and enablers for continuous bioprocessing have been considered.
Chen noted that the challenges of using disposable bioreactors are the current limitation in size, with 4,000 liters being the maximum, and the need for manual installation, which involves tough training requirements. Some cell lines are sensitive to extractables and leachables and require selecting clones that perform well in bags. WuXi Biologics has selected a scale-out strategy based on 2,000 liters to help the company rapidly adjust production to various stages in the life cycle and meet unpredictable market demands. The risks associated with cell culture scale-up are eliminated by this approach. Furthermore, the risk of market supply interruption can be minimized by a dual sourcing for supplies. Technology transfer will be much easier, too.
Chen stated that there are limits for bioreactor scale-up to very large scales (from 10,000 to 25,000 liters). He also explained that the forces driving continuous processing include the need for flexibility and speed to market, the demand for more low-volume biologics, business competition, and the desire to restrain the cost of goods.
Mary Harney shared her perspective as a long-time government leader. Over 18 years, she held several ministerial positions in the Irish government, including in the areas of environmental protection, industry, trade, research and innovation, and health. She was deputy prime minister for 10 years and was both the longest-serving woman in the Irish Parliament and the longest-serving female minister.
Harney pointed out that the presence of pharmaceutical plants and technical operations in Ireland is considered a real asset for the country. In general, industry should be proud of driving innovation and creating value for patients in health and disease management, she said.
Thomas Friedli, Associate Professor and Senior Lecturer of Management at the University of St. Gallen, Switzerland, explained that in recent history, all major pharmaceutical companies have established their own production systems based on the groundbreaking Toyota production system. Nevertheless, companies still face difficulties when they try to quantify the influence of the different system elements on overall performance.
In growing numbers, companies are interested in research on this issue to determine their strongest strategies for their production systems and assign resources accordingly. Using data to further accelerate continuous improvements is essential. In a digitalized industry, more and better data are available for such analysis.
Friedli noted that 94% of companies don’t believe that digitalization will make Lean-style management redundant. Also, although digitalization seems easy in theory (and in consulting practice), there are many conceptual challenges to overcome. Therefore, real success stories depend on careful consideration of where “digital” can make a difference. One prerequisite for a successful transformation to industry 4.0 is a plant technology competence framework model, which includes three basic qualifiers: access to skills and knowledge, access to low-cost production, and proximity to market. Decisive factors in this model are automation and manufacturing; human-machine interfaces; data analysis and information and communication technology (ICT); embedded systems; and assurance that activity regarding technology X is also performed for other sites in the manufacturing network.
In conclusion, Friedli recommended that digitalization should be understood to be an opportunity to increase efficiency and improve quality while also taking its complexity into consideration. After having spent some time on an emergent approach, the pharmaceutical industry should move toward the deliberate planning and implementation of digital technologies.
In his presentation, Graham Cook, Senior Director of Process Knowledge/Quality by Design at Pfizer and European Federation of Pharmaceutical Industries and Associations (EFPIA) topic lead for the International Conference on Harmonisation (ICH) Q12 standard answered the question, “Why cover ICH standards in an executive forum?”
He explained that the pharmaceutical industry is the most regulated industry globally, and regulatory standards are essential to ensure health and safety and form a strong foundation for regulatory oversight. To uphold the ICH Q12 pharmaceutical standard, company management is responsible for knowing the principles in the standard and allocating adequate resources to uphold them.
The product life-cycle management components of ICH Q12 can be considered an innovation in regulatory standards with regard to the following elements of postapproval changes (PACs), which can be used for new and existing products:
- Established conditions
- PAC management protocol
- PACs for marketed products, including a structured approach to analytical procedure changes and stability data approaches to support the evaluation of changes in chemistry, manufacturing, and controls
Cook emphasized that ICH Q12 is already serving as an agent for change within regulatory agencies to simplify PAC management and encouraged management and senior experts to “learn by doing.”
A positive surprise came when Cook asked the audience, “Did you read the ICH Q12 draft?” and a considerably large number of attendees raised their hands.
Innovation, at all levels and for all stakeholders, is key to success.
Keynotes
The keynote addresses on Tuesday, 2 April, were given by high-level representatives of large manufacturers. These speakers shared their companies’ approaches to innovation in the small molecule and biopharma business.
As an introduction, John Bournas, ISPE President and CEO, noted positive developments in ISPE’s conferences, membership, training, and publications. In particular, Bournas said attendance at the Europe Annual Conference has grown significantly since the first conference in 2014.
In his keynote address, Brendan O’Callaghan, Senior Vice President and Global Head, Biologics Platform, at Sanofi, noted that Sanofi’s manufacturing strategy is evolving to meet the needs of a growing and increasingly divergent biologics pipeline. The company’s overall goals are speed to market, flexible/agile and reliable supply solutions, competitive costs of goods, and better service to the needs of patients. Digital technology is expected to transform the way that therapies are discovered, developed, and delivered to patients, providers, and payers.
The company’s operational strategies are focused on large-scale cell culture (microbial and mammalian). Strategic partnerships will provide additional capacity and optionalities as well as a future platform for smaller scale, single-use, and digitally enabled technology. Furthermore, this platform should be “product agnostic” to enable a diverse pipeline.
For some time now, 70% of portfolios have been represented by biologics. Research in a broad range of sciences is being conducted to select the right tool for the right target. Areas of investigation include antibody conjugates, peptides, enzymes, fusion proteins, multispecific antibodies, small molecules, nanobodies, gene therapy, gene editing, and mRNA and siRNA conjugates.

Jim Breen, Vice President, Lead Biologic Expansion, Janssen Pharmaceutical, and 2019 ISPE International Board of Directors Chair, discussed trends and disruptors that Johnson & Johnson is following in healthcare. In particular, the company is focused on consumers and patients seeking personalized and on-demand experiences, marketplace consolidations, middle-class expansion in emerging markets, and the growth of an aging population.
Breen emphasized the challenge of creating “intelligent” manufacturing systems in which the production system is able to predict quality, output, service, and maintenance via self-learning and self-correcting mechanisms. Meeting this challenge will require connecting the manufacturing infrastructure, equipment, materials, and people through data systems.
Rick Friedman, Deputy Director, Science and Regulatory Policy, Office of Manufacturing Quality, FDA/Center for Drug Evaluation and Research (CDER), focused on recent regulatory findings and sustainable solutions in the context of Pharma 4.0™. He pointed out that quality risk management (QRM) is not sustainable or effective if it is static. The QRM program should support continual improvement because QRM is an iterative, life-cycle endeavor. Notably more information is available after product launch and through extensive batch production experience than was available in early experiments. This new knowledge inevitably identifies new QRM opportunities. The links between QRM and knowledge management (KM) involve many factors such as complaints, rejections, and feedback from the shop floor; maintenance issues; deviations; returned goods; out-of-specification and stability results; current staff competencies; raw material data; results of audits; and process trending.
Pharma 4.0™ approaches will help to connect the dots between these factors to present a holistic view on product life cycles. Data integration and pattern recognition are critical for managing quality risks, he said. Human-factor risks during production can be effectively reduced.
In summary, the current pharmaceutical quality system drives sound life-cycle decision-making, which is based on a strong QRM and KM foundation. Furthermore, it establishes and maintains a state of process control; sources robust materials from qualified vendors; designs reliable tests, processes, and products; monitors process performance and product quality; and manages risks effectively. It creates real, long-term, and systemic solutions for problems.
Joydeep Ganguly, Senior Vice President of Operations at Gilead, focused on execution of Pharma 4.0™ from a practitioner’s perspective, using the following elements to describe its realization:
- Collaboration
- Foster communities with research and development
- Reimagine the notion of technology transfer
- Link space plans to scientific flow
- Implement a socialized collective vision throughout the value chain
- Create an open space that can be adjusted to fit different purposes
- Create technology-enabled collaboration zones within a campus to allow for an academic campus atmosphere
- Create laboratories with a greater digital footprint
- Connection
- Shift to cloud computing
- Introduce and assimilate social tools
- Increase and enhance web and video conferencing
- Leverage technology to drive a culture of co-creation
- Control
- Implement an automation concept to drive a disciplined approach to data governance and structure
- Increase point-of-use analytics to put data in the hands of users and automate a multitude of reports
- Develop a life-cycle approach
- Use analytics cases to increase predictive power within facilities
- Safety
- Conduct risk assessments to guide safety constructs within facilities
- Increase technology footprint to reduce risks in processing and operations
- Employ advanced analytics to drive a reshaping of campus pathways, signage, lighting, and vehicular flow, thereby promoting a new culture of safety-by-design
- Harness the power of robotics and predictive analytics to create more sophisticated near-miss surrogates
- Security
- Implement a sophisticated security system that leverages the combination of people analytics, social media, and ecosystem changes to assess and track risk
- Collect real-time analytics on campus risk
- Pilot all-based tools to create robust investigations and assessments
- Automate an overall crisis management plan to enable a mobile solution for the overall risk and emergency process
- Sustainability
- Prioritize energy-efficient operations
- Encourage eco-friendly behaviors
- Conserve resources, such as using wind, solar, and fuel cells to provide 100% of power
| Plasma & Biologics | Small molecule dosage forms |
|
|---|---|---|
| Dose regimen | Daily to (bi)weekly | Daily |
| DP Size (# Packs) | Medium to low (thousands) |
High (millions |
| Batches of 1 Product /year |
< 50 Batches | > 100 Batches |
| Products per Line/ year | 2-3 | > 10 |
| Target DP Stability | 2 years | 3- 5 years |
| Flexibility | Low | High |
| Disposables Steps | High | Low |
| Capex | High | Low to medium |
Source: Thomas Wozniewski, Takeda
ISPE’s Women in Pharma® Initiative delivered a panel discussion on emerging innovations in the pharma industry. The panel was led by Susan Hynes, Vice President and Site Lead for Takeda Dunboyne Biologics; Bernadette Doyle, Vice President of GSK corporate technical and NPI pharma supply chain; and Amy White, Janssen Sciences UC Senior Operations Manager.
The discussion focused on agile principles for team design, showing all elements of Pharma 4.0™ in a holistic manner. This approach is a structured one, starting with strategy and a roadmap, followed by organization and governance, leadership and culture, and needed capabilities, and closing with enterprise business processes. The panel members emphasized that Pharma 4.0™ is far more than a technology project driven by digitization.
Hynes noted that digital enables agile. Doyle highlighted digital enterprise skill sets and tools such as agile learning, thinking in systems and from the enterprise point of view, and breaking away from silos. White discussed the operations perspective by addressing needs for process intensification, modularization, automation, and patient-centered solutions.

Thomas Wozniewski, Global Manufacturing and Supply Officer at Takeda, presented on the Transformation of Corporate Manufacturing and Supply at Takeda. This was a highlight of the conference. Using the example of the gene and cell therapy product Alofisel, he demonstrated the high complexity of manufacturing and supply of this new generation of products.
The main stages in the end-to-end supply chain are mobilized blood cell collection, selected stem cells, which are transduced with the selected gene, harvested, and cryopreserved, and application by infusion to the patient.
Several factors make the production and delivery of the product challenging, including the 48-hour shelf life, which requires tracking the expiry time as well as the expiry date; storage conditions of between 15°C and 25°C; and the need for direct distribution from the plant in Madrid to about 150 treatment centers in Europe, Israel, and Canada, with transit times of up to 22 hours. In addition, production of the treatment starts seven days prior to surgery, and one batch of four vials is designated for only one patient.
Clearly, time is of the essence, and only digitalized supply chains can solve such a complex delivery challenge. The goal is to match the treatment day with the delivery day on a platform that all stakeholders in the supply chain can use to collaborate. The availability of daily production capacity is disclosed to nurses. Dedicated and specialized logistics service providers and qualified transport lanes and shippers are utilized. Everything is supported by a strict GxP protocol for treatment receipt and checks. As a result, customer service levels higher than 99% have been achieved.
Wozniewski also highlighted the following major industry trends:
- Changing technologies: Different modalities require different approaches (see Figure 3).
- Drug product and medical device combinations: For example, Factor VIII treatment is being optimized and personalized through these types of products.
- Changes in the regulatory framework that result in shorter development time: For example, certain innovative drugs for rare diseases, oncology products, and personalized medicines are being fast-tracked for approval.
- Environmental sustainability: Consumers, governments, investors, and others are increasing the momentum for environmental protection measures. As a consequence, company commitments to sustainability and public positions on related social and political issues are expanding.
Small batch sizes are becoming more common, driven by biosimilar production, personalized medicine, and higher monoclonal antibody titers.
Other Events
After the keynote session, the conference was divided into four tracks: Facilities of the Future, Pharma 4.0™, Quality and Regulatory, and Project Engineering. The speakers (about 70 in all) delivered high-quality presentations and engaged audiences in question-and-answer sessions and panel discussions. Attendees appreciated the opportunity to interact with several actively involved regulators from various countries, and the exhibit halls were filled with 76 tabletops and booths sponsored by machine, equipment, infrastructure, digital technology, and consultancy companies, and supply chain management providers.
Overall, the feedback on the conference was extremely positive. Many participants have already committed to attend ISPE’s 2020 Europe Annual Conference, which will be held 30 March through 1 April in Madrid, Spain.

Attend the 2020 ISPE Europe Annual Conference in Madrid, Spain, 30 March – 1 April.








