Designing new facilities for cell and gene therapy manufacturing is a challenging task given the many uncertainties in this industry sector, including varying potential demand for any given new therapy, evolving platforms and technology, questions about equipment reliability, learning curves for analysts and operators, possible sourcing issues, and variable lead times for key raw materials....

Downloads
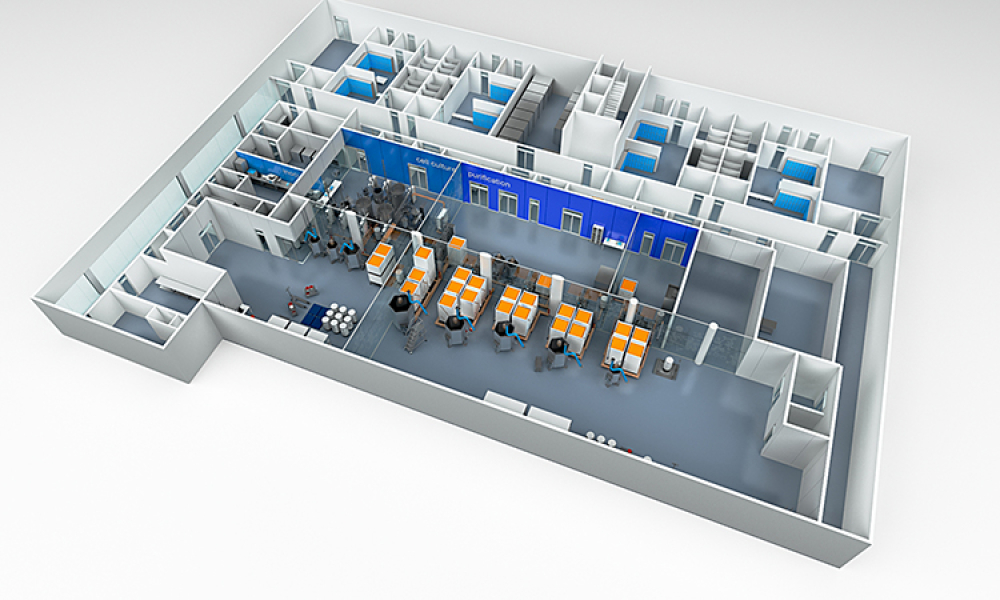
Cell and Gene Therapy Facility Design Using Simulations
Cover: Designing new facilities for cell and gene therapy manufacturing is a challenging task given the many uncertainties in this industry sector. One approach to managing these uncertainties at the facility design stage is to develop operational models and perform computer simulations. The information generated via these simulations enables management to make data-driven decisions.
Flexible Facility Design for Multiple Cell Therapy Processes
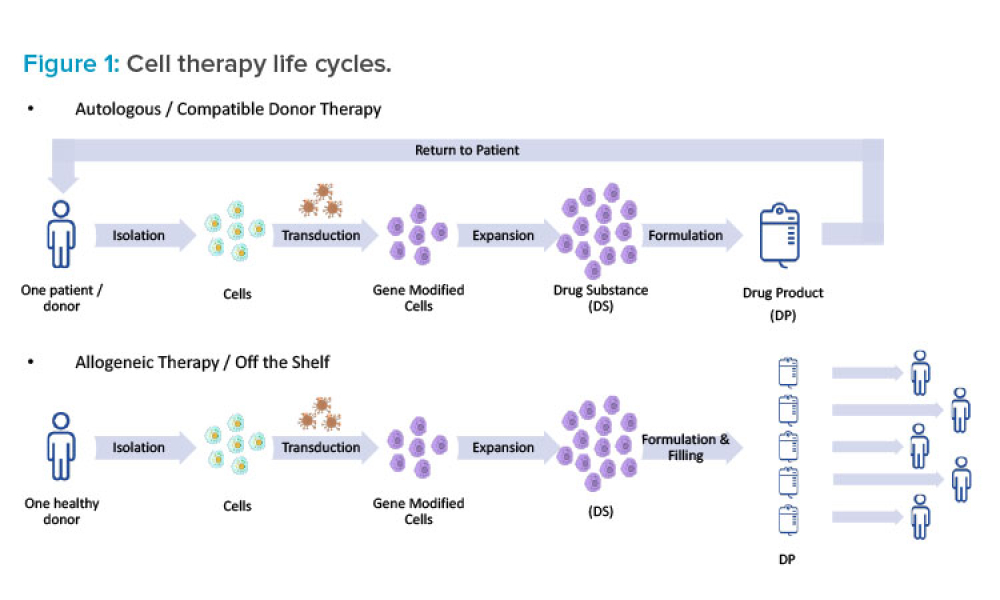
Feature: This article surveys several of the key issues to consider when designing facilities capable of manufacturing multiple cell therapies, including regulatory de nitions, product life cycles, processing systems, relevant cell therapy technologies and equipment, biosafety, cross contamination, facility automation, and layout options.
Current State of Oligonucleotide Therapeutics
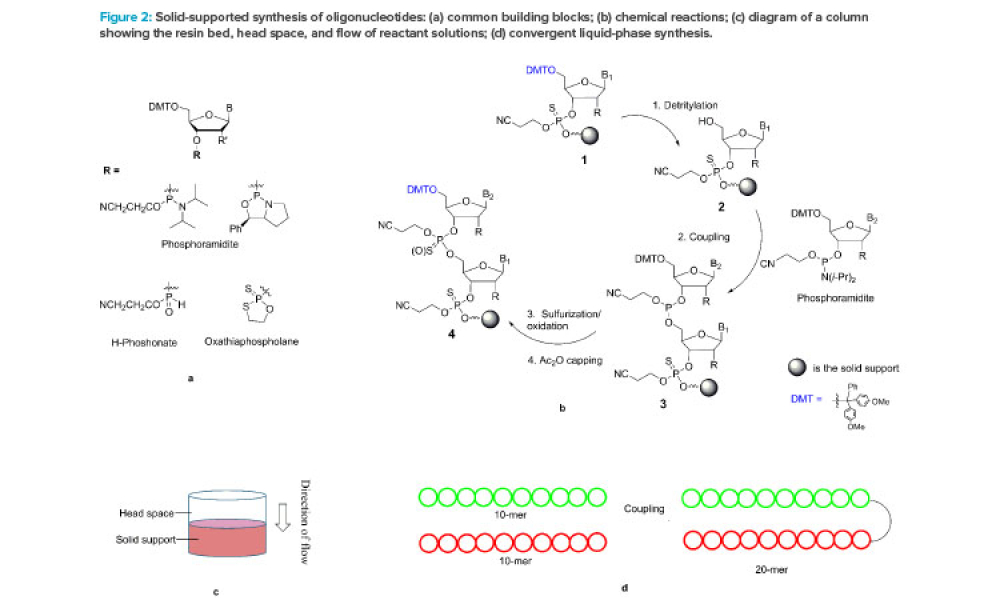
Feature: Oligonucleotides are a relatively new class of drugs, composed of natural and synthetic nucleotides. This article focuses on synthetic antisense oligonucleotides (single strand 16–22 mers) and small interfering RNA (double strands formed by hybridization of a pair of complementary sense and antisense strands, 19–25 mers).
2019 ISPE Europe Biotechnology Conference: Industry Transformations
Feature: Biopharmaceuticals have brought significant transformations in product development, strategy, technology, and operations. This ongoing transformational process was the main theme of the fourth annual ISPE Europe Biotechnology Conference, 25–26 September 2019, in Brussels.
Justifying Investment in Manufacturing Execution Systems
Technical: Justifying Investment in Manufacturing Execution Systems Investments in manufacturing execution system (MES) applications may reduce costs and increase revenues, but they also might compete with other investment priorities. This article offers guidance for life sciences companies considering investment in MES applications, including how to measure MES-related cash flow and reasonably evaluate an investment in MES versus other alternatives.
In This Issue
2020 has been a super-busy year for ISPE Women in Pharma® (WIP). We started 2020 with aggressive goals, including to set up global Mentor Circles; start a monthly Women in Pharma® newsletter, The Bridge;...
As this is my last Young Professional (YP) column for Pharmaceutical Engineering®, I wanted to start with a quote from Winston Churchill that I feel summarizes my two-year journey as the International Young Professional Chair: “Success is not final, failure is not fatal: it is the courage to continue that counts.”
Given the events that have transpired over the last 12 months, I have penned the title of my final column as ISPE Chair to reflect the temperament of our times. Coincidentally, and please pardon my digression, the title also happens to be the name of a hit album of topical songs by Tom Lehrer, recorded live in July of 1965 at the “Hungry I” nightclub in San Francisco, California.
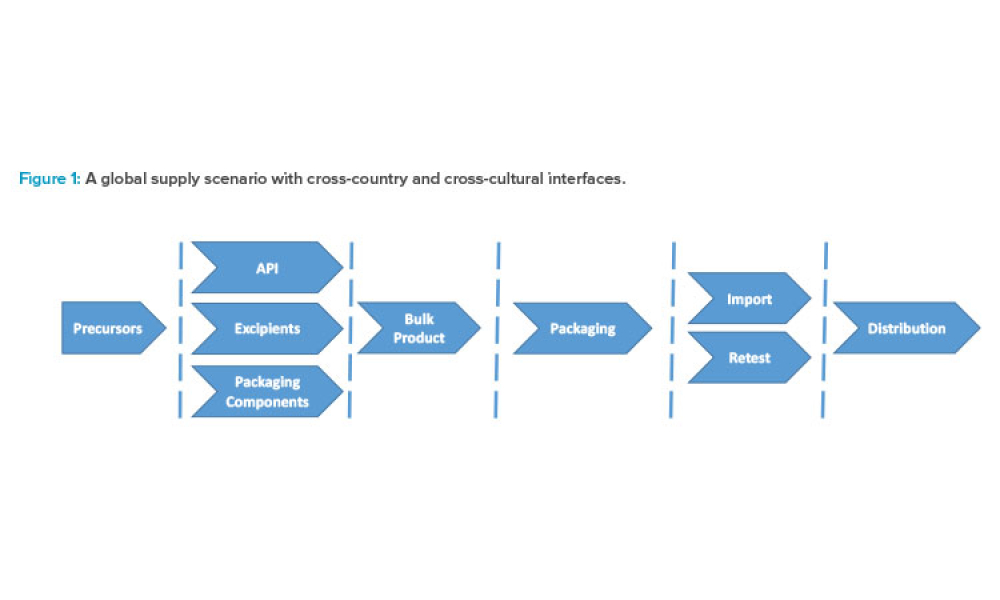
The current regulatory framework in the pharmaceutical industry places pressure on marketing authorization holders (MAHs) to demonstrate quality oversight, and a systematic risk management process is a prerequisite for avoiding compliance and productivity pitfalls. This article focuses on options to improve day to- day operations and to ease decision-making by integrating operational risk...
In many critical ways, the design of facilities for multiple cell therapy processes is unlike the design of conventional pharmaceutical facilities. This article surveys several of the key issues to consider when designing facilities capable of manufacturing multiple cell therapies, including regulatory definitions, product life cycles, processing systems, relevant cell therapy technologies and...
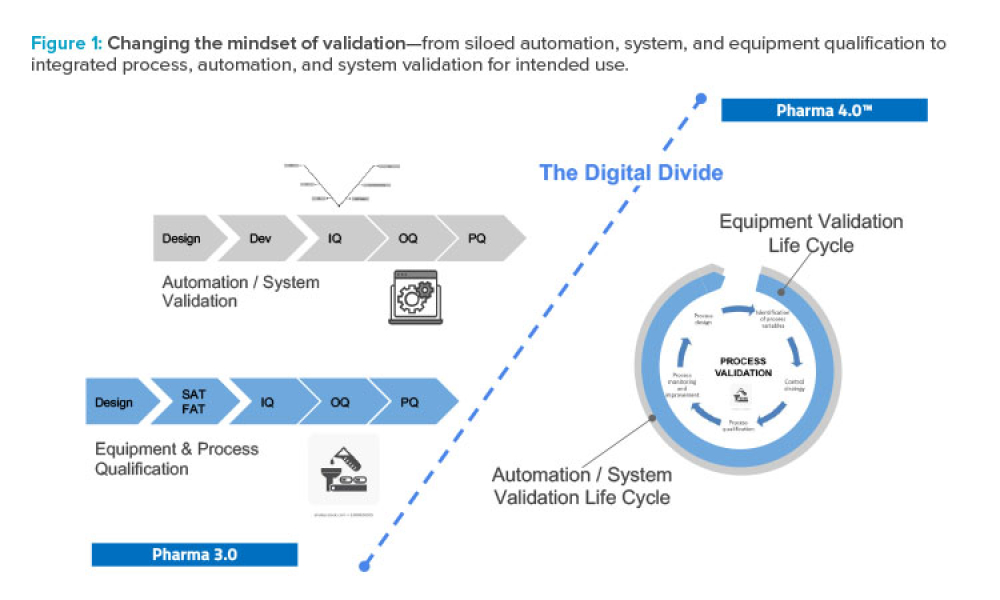
As Pharma 4.0™ increasingly becomes reality, our validation practices must change. We can no longer apply 20th-century thinking to 21st-century technology and resources. Validation must adapt to industry shifts from iterative to...
Oligonucleotides are a relatively new class of drugs, composed of natural and synthetic nucleotides, which primarily include small interfering RNA (siRNA), micro RNA (miRNA), and antisense oligonucleotide (ASO). These molecules achieve therapeutic effects through RNA interference, degradation, or splice-modulating pathways.1
ISPE is celebrating its 40th anniversary this year! Here are some of the memorable events from the last 40 years.
The ISPE D/A/CH affiliate Is a Leader in Many Respects, from Its Ever-Increasing Size to the Invaluable Engagement of Its Members, Who Contribute to Ispe’s Growth around the World. Grouping Together Europe’s German-Speaking Countries—Germany (D), Austria (a), and Switzerland (Ch)—It Is Currently the Second Largest Ispe...
To continue meeting the professional needs of ISPE members and with the global increase of virtual events due to COVID, ISPE has expanded its webinar offerings on topics important to ISPE members and industry sponsors.
Executives at manufacturing companies of all sizes need to make decisions about where to invest to maintain and grow their businesses. Investments in manufacturing execution system (MES) applications may reduce costs and increase revenues, but they also might compete with other investment priorities, such as marketing campaigns and capital equipment upgrades. This article offers guidance for...
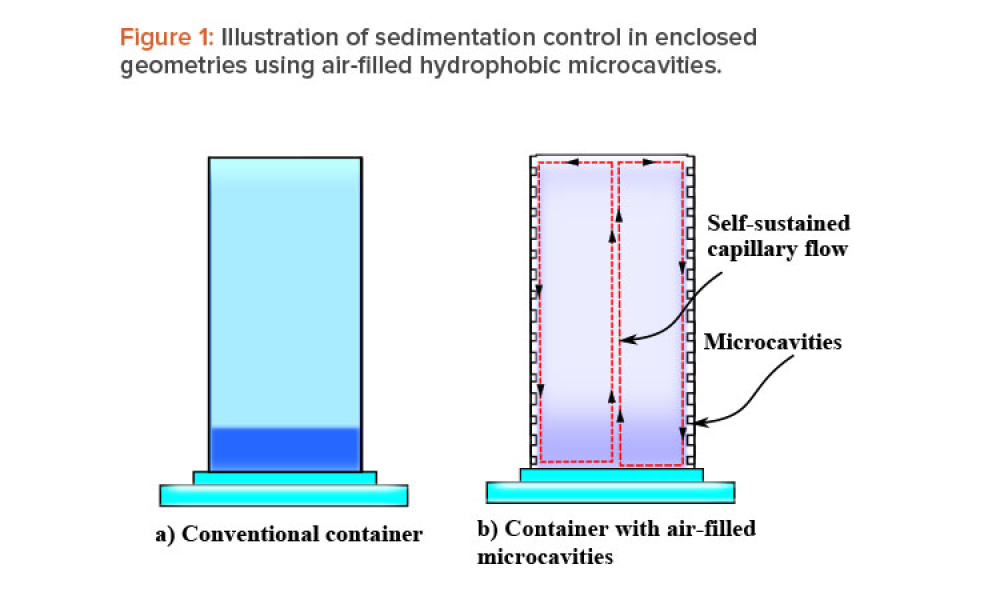
When fluid-filled containers are stored for long periods of time with negligible motion, sedimentation and gravitational settling of particles can occur. This article discusses a passive sedimentation control mechanism that is driven by Marangoni stress, which is induced in enclosed geometries when their walls are lined with gas-filled hydrophobic microcavities.
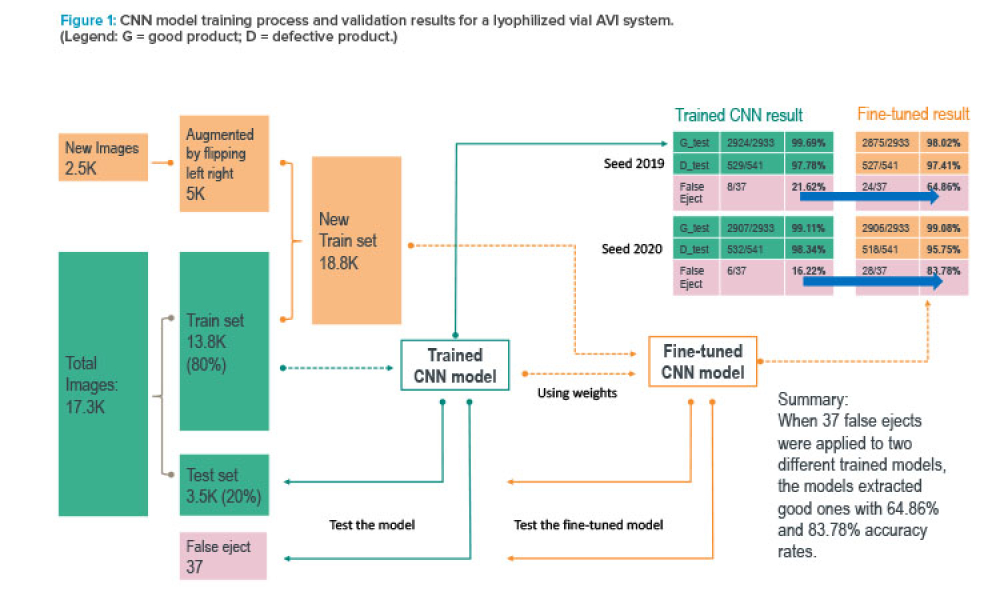
Pharmaceutical companies rely on automated vision inspection (AVI) systems to help ensure product safety. Although these systems overcome challenges associated with manual inspection, they can be hindered by limitations in their programming—if the system is programmed to consider every variation in inspection conditions, it is likely to falsely identify defects in safe products. This article...
Joydeep Ganguly, who is currently Senior Vice President, Corporate Operations, at Gilead Sciences, Inc., places a premium on working for a mission-oriented company. “If you choose the right company with the right ethos and a deep, tangible commitment to the patients, the mission is not just rhetoric,” he said. “You see it in the way they do things. If you work for a company that makes that...