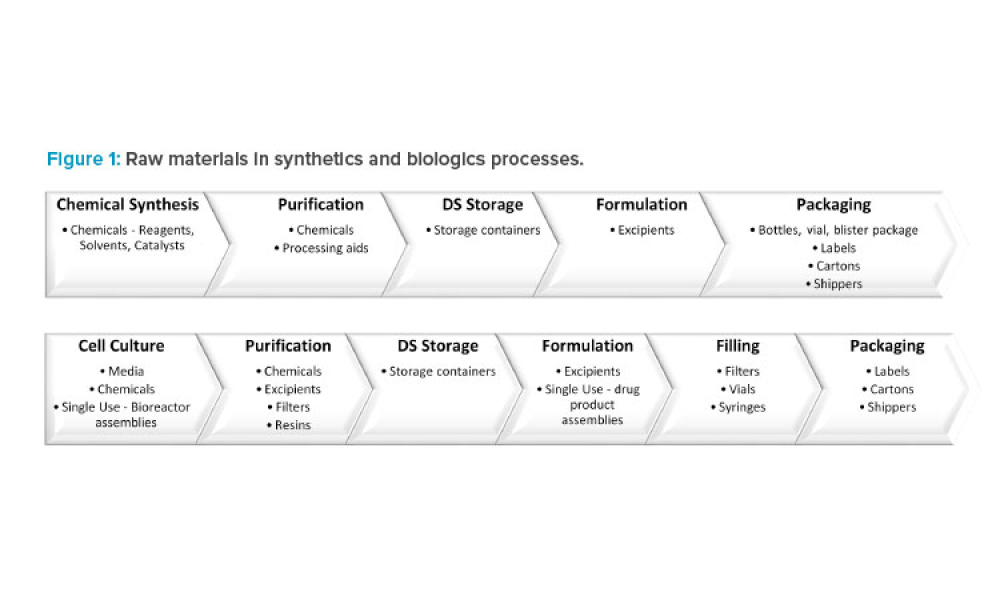
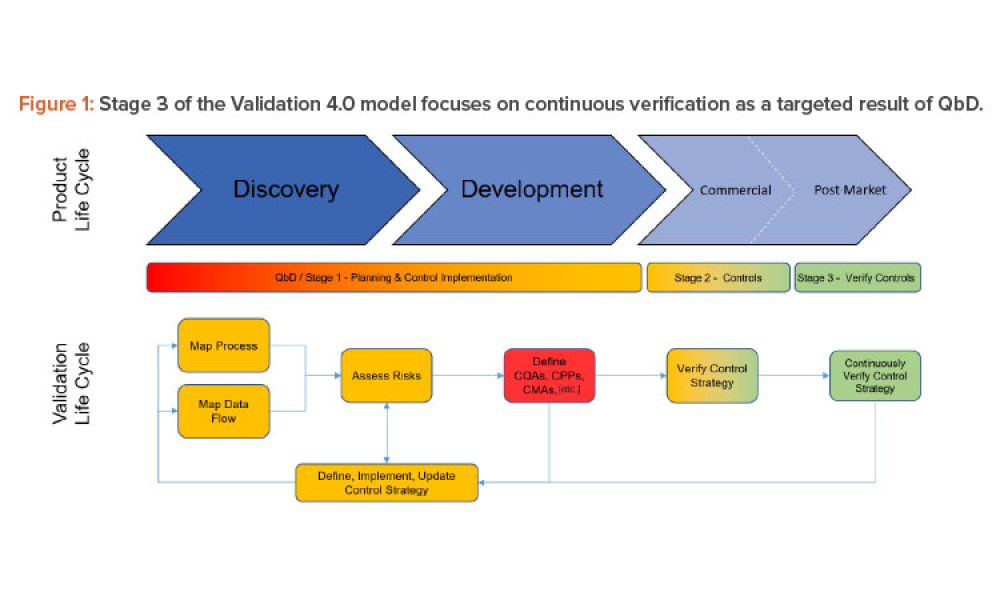
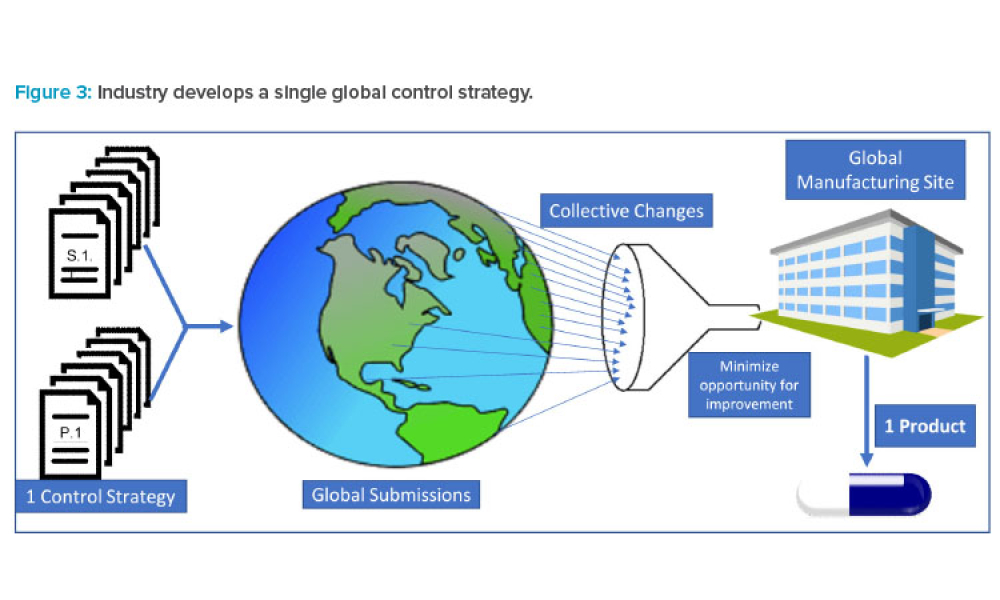
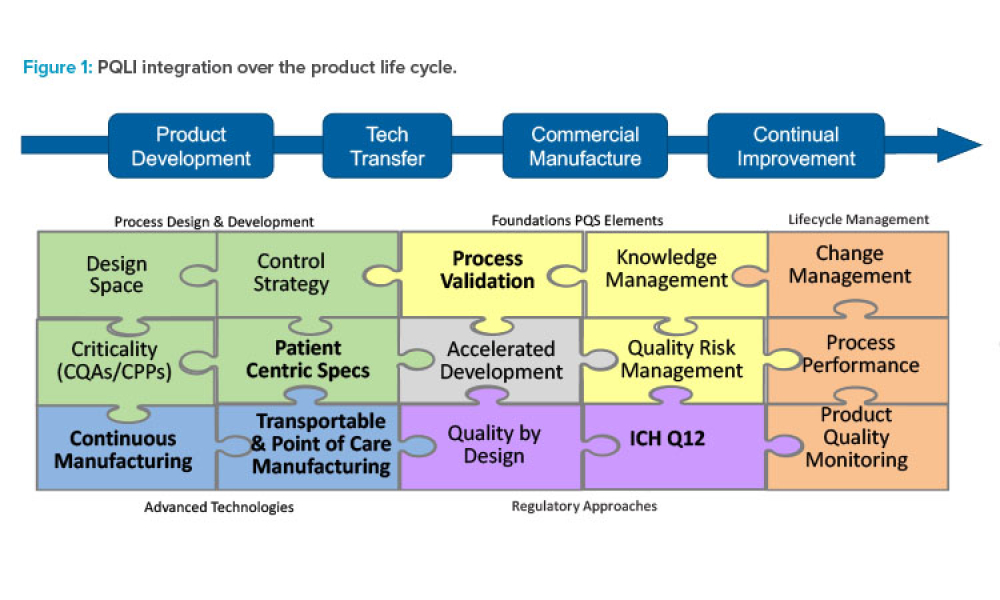
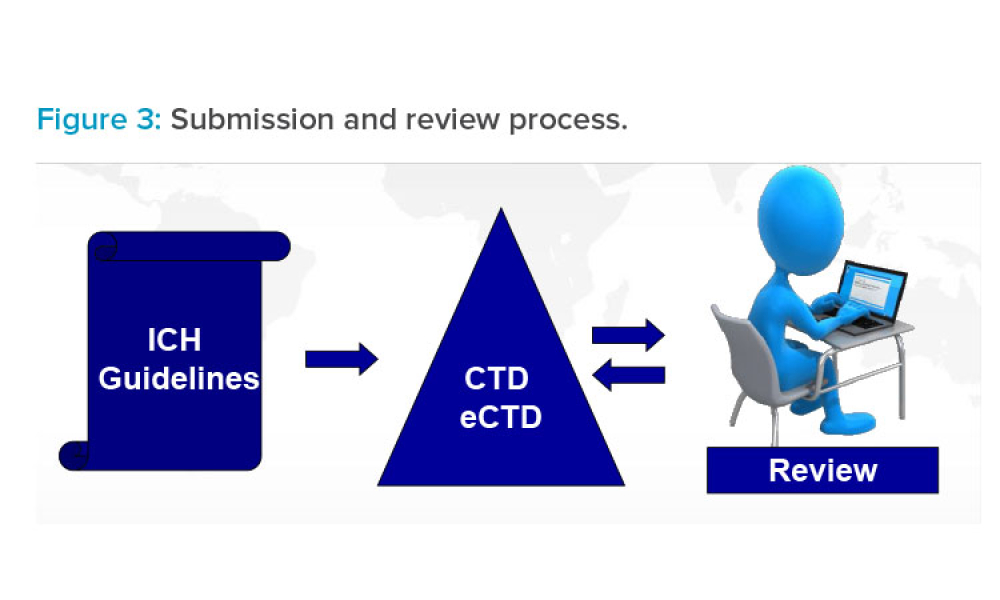
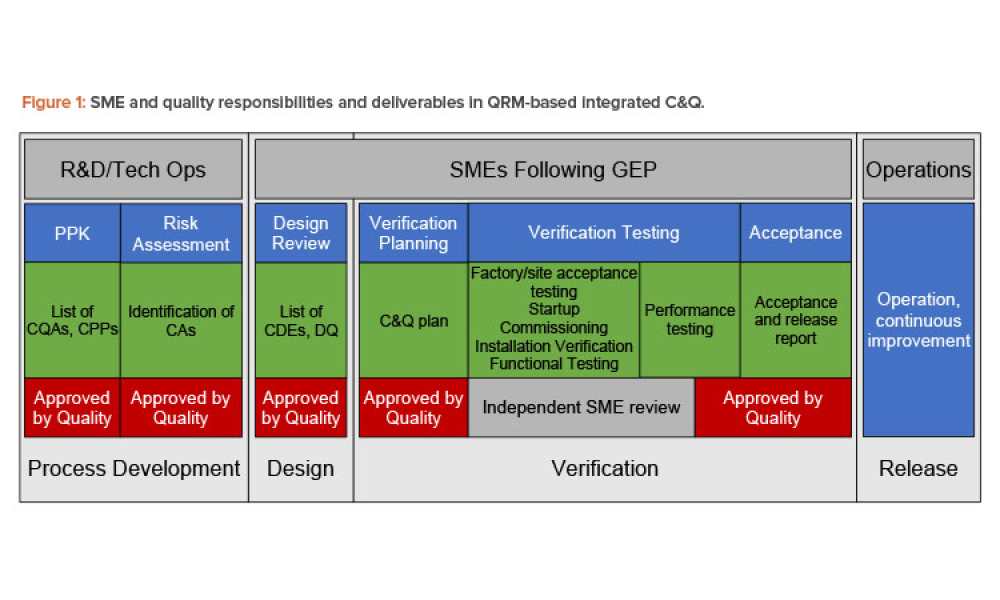
In 2011, the US Food and Drug Administration (FDA) introduced the revised “Guidance for Industry: Process Validation: General Principles and Practices.”1 The document incorporated...
ISPE’s regulatory initiatives and programs bring visibility and solutions to significant regulatory and quality challenges faced by the industry, facilitate the flow of information between ISPE members and global health authorities to find solutions, and promote interaction between regulatory bodies in the interests of harmonization.