Automation
There is a significant need for plug-and-play software. However, because of security concerns, systems must be modified to be operated by remote inputs and outputs (I/O) and an overall process control system. This has contributed to delays in progress in this area, and can result in unnecessarily high costs for the facility owner.
The BioPhorum Operations Group (BPOG) automation team is actively addressing these challenges and expects plug-and-play bioreactors to be available in 2021.
Buffer Storage versus Just-in-Time Preparation
The only place where just-in-time (JIT) preparation has been routinely used is in the filling of bioreactors. The resistance to JIT buffers seems to be mainly due to the need to have buffers in place before starting the purification step. The one relatively routine exception to this is sodium hydroxide, where the use of storage tanks and loops is more commonplace.
The Wheels Are Not Removed
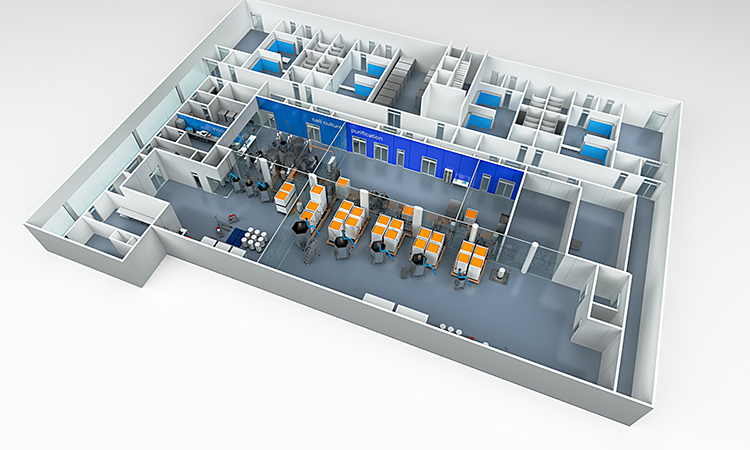
The concept of clustering equipment was broached in the original article, and a good example of this approach can be seen on the front cover of the ISPE Good Practice Guide: Single-Use Technology
In effect, the equipment is not bolted to the frame/platform, allowing for easy removal, if required, while at the same time providing support for GMP trunking and utility pipework. There is also the added advantage of reducing the number of utility drops.
Equipment clustering also provides a degree of freedom because the order of equipment in the production train is not necessarily fixed. For example, if an additional purification step is required, it can be easily incorporated through clustering.
Another area in which clustering is advantageous is in multiproduct facilities where each product has a dedicated production train configuration. With equipment clustering, the reconfiguration and production space turnaround can be greatly reduced.
The Future
Buffer Management Strategy
There have been many advances in technologies for upstream and downstream process steps over the last few years. And now, as demonstrated in a recent BPOG paper, some of these technologies, such as inline dilution and inline conditioning, can be adopted for use in the preparation of buffer solutions.
A buffer management strategy that blends conventional and new technologies allows the footprint required for buffer preparation to be reduced. This means a smaller overall facility footprint in new facilities, and an additional area in existing facilities that could be converted into production space.
One notable example of this is Kedrion Biopharma, which achieved space savings of 61% by implementing inline conditioning. This space saving refers to production space and is a reduction of space compared to traditional buffer preparation methods.
As buffer preparation areas are reduced in size, a preparation suite that would have been dedicated to one production area in the past could now support multiple production units. This opens up new possibilities to increase production and expand facilities.
Modular and Prototypical Design
Although the time to design and construct production facilities has been dramatically reduced, facility design and construction cannot keep pace with the daily evolution of technologies and new medicines. The use of a prototypical or model design and modular approach has the potential to reduce these times significantly. A prototypical design is a master design, one that can be replicated at various sites and facilities. In addition to reducing facility design and construction time, it also has the benefit of reduced operator training, as all facilities are the same. It also has the potential to reduce the time for commissioning activities, as many protocols and other documentation can be leveraged from the prototype facility.
Robots Don’t Dance
At first glance, there do not seem to be many applications where modern robotics could be used in a single-use facility; loading a 2,000-liter bioreactor bag would not be something suited to a currently available robot. ,
To see how robots will be employed in biopharma, we need to look at the bigger picture of all production-related activities, such as laboratory and logistical functions, and not just core manufacturing tasks around the unit operations.
In a recent 1,000-liter, single-use continuous facility, management realized that the facility could operate with most of its manufacturing staff working from 8:00 until 17:30. This was due to the ability of the control system to run overnight without any manual interventions. The change in staff hours significantly reduced the manufacturing head count. However, quality control and warehouse operations were largely unaffected. Mobile and static robotics applications are now being investigated to take over repetitive tasks in those departments. Areas of interest include:
- Environmental monitoring (see Figure 3)
- Water sampling
- Product sampling
- Sample transfer
- Subaliquot
- Insertion of samples into analytical equipment
- Kitting of materials
- Movement of materials