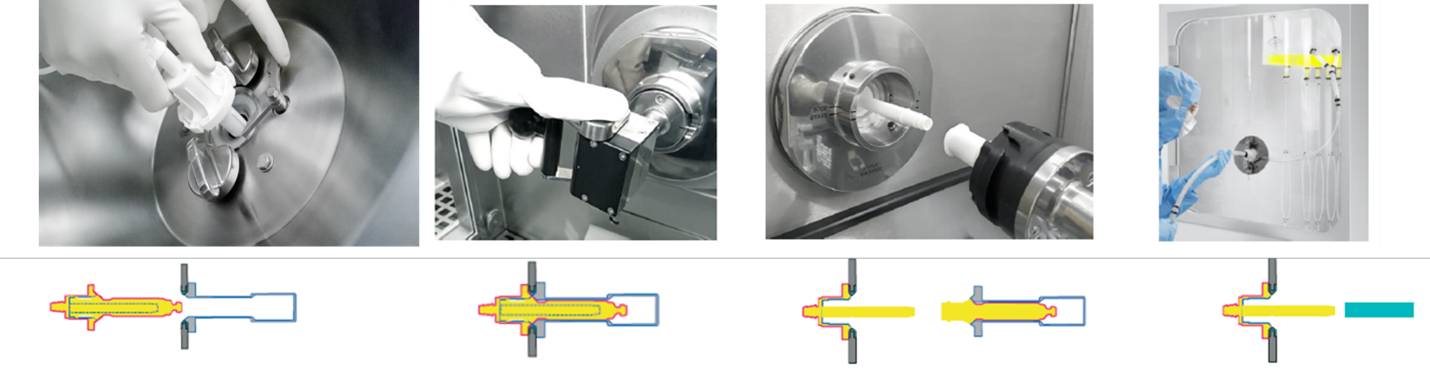
What should be considered with closed systems concerning single used systems (SUS)? SUS are widely used in pharmaceutical manufacturing, primarily in biopharmaceutical manufacturing and upstream and downstream area. They can be single-use bioreactors, filter systems, and etc. The different process steps are then connected with so-called aseptic connectors—or sterile connectors. The entire process is carried out as a closed system.
The following aspects should be considered for a successful installation of the SUS:
It is important to consider that SUS can easily be damaged because of their flexible design, so they should be packaged and stored correctly (storage on top of each other should be avoided). Unpacking and assembly can also lead to damage. When dismantling a SUS, care must be taken not to break the containment and contaminate the room.
Since single-use systems are not cleaned after their use but disposed of, there is no need for time-consuming cleaning as with rigid installations. This allows a quicker switch to the following product. If the biopharmaceutical product is a highly active substance or the substance requires a higher biosafety level such as BSL 2, or if viral vectors are being handled, it is advisable to take critical process steps to prevent possible contamination of the environment. For example, by dismantling the SUS and installing it into another barrier such as an isolator. Also, the isolator can form an aseptic barrier around the single-use system if the integrity of the single-use system cannot be assured.
Isolators are seen as another closed system. These are now divided into two categories in the new Annex 1:
- Closed isolators that can be installed in clean room class D, and
- Open isolators that can then be installed in clean room Class C.
But what is the difference between a closed isolator and an open isolator?
Definition from the EU GMP Draft Annex 1 Version 12:
Closed isolator systems exclude external contamination of the isolator’s interior by accomplishing material transfer via aseptic connection to auxiliary equipment, rather than use of openings to the surrounding environment. Closed systems remain sealed throughout operations.
See picture of a closed Isolator below for the use in Cell and Gene Therapy.
Open isolator systems are designed to allow for the continuous or semi-continuous ingress and/or egress of materials during operations through one or more openings. Openings are engineered (e.g., using continuous overpressure) to exclude the entry of external contaminant into the isolator.