A New Regulatory Approach to Drive Sustainable Medicines

To enable changes across the pharmaceutical industry, sustainability should be included alongside quality, efficacy, and safety when assessing medicines. This article reviews two case studies that cover sustainable pack types and extension of shelf life. With the drive to manage unmet medical need through acceleration of drug development programs, postapproval sustainability variations will always be required. Here we discuss if current regulations will be fit for a sustainable future.
For decades, the pharmaceutical industry has worked to transform the lives of patients by researching, developing, and manufacturing medicines for a wide variety of common and rare diseases, something that will continue for many years to come.1 Now there’s an additional focus: sustainability. The implementation of sustainability-driven initiatives associated with the manufacture of medicines faces many challenges from a chemistry, manufacturing, and controls (CMC) regulatory point of view. The regulatory procedures and data requirements make it very complex to improve sustainability for launched products compared to building sustainability into new products during development.
As such there’s a growing question of how the industry will improve the sustainability profile of its existing medicines and ensure that sustainability is designed into new medicines, such as products, with a reduced environmental risk, greener manufacturing technologies, and recyclable delivery systems and packaging.2 With the pharmaceutical industry being such a major contributor to the global economy and impacting the lives of so many,3 the industry finds itself under the spotlight of expectation to give a higher priority to sustainability initiatives.
In order to provide innovative solutions for embedding sustainability into products, industry requires collaborative assistance from global regulators to allow faster implementation of sustainability initiatives by using risk-based scientific approaches as described by ICH Q124 and driving harmonization across regulators globally. Global regulators are assessing drug products for quality, efficacy, and safety. To enable changes across the industry, sustainability should be included alongside these.
This article provide insights from a CMC regulatory perspective into what is required for the pharmaceutical industry to develop and manufacture sustainable medicines which minimize the impact on the environment, utilizing two case studies based on real-world experience.
Case Study One: Packaging Materials
The first case study looks at developing more sustainable packaging materials and reducing the size of packaging materials. Industry invests significant effort into designing sustainability into the development of new medicines. But what happens when these sustainability-driven options are not developed, or available, to meet the timelines of launching new products for patients, and what about the increasing drive to improve the sustainability profiles of existing medicines that have been, and will continue to be, marketed for many years?
Let’s use the example of the packaging for medicines. Modifications to the primary and secondary packaging would be considered for a number of sustainability-driven reasons, such as to:
- Decrease material consumption and wastage by reducing the primary packaging dimensions
- Further material savings in the secondary packaging due to reduced primary pack dimensions
- Improve shipping efficiency with reduced secondary packaging dimensions
- Move toward more environmental-friendly or recyclable materials in primary and secondary packaging
As part of the development program for a new medicine, the onus is on the pharmaceutical companies to create sustainable packaging solutions during the development program and have data to support its use available in time for registration. This facilitates launching the new medicine with the desired packaging and meeting the sustainability objectives. The exact data requirements are dependent on the pharmaceutical product. However, this generally equates to a certain amount of real-time stability data in the proposed commercial pack, with the shelf life granted at registration depending on the length of real-time data available, as exemplified in Table 1. It is worth noting that at the time of initial registration, supply chains can often be simpler than those of established commercial products because the new product has yet to undergo brand growth, globalization, and invest in maximizing supply as new indications are introduced.
| New Product in Development | ||
|---|---|---|
| Product Details | 2 strengths for global launch | 2 strengths marketed globally |
| Supply Chain | Simple: • 1 formulation site • 1 packing site |
Complex: • 3 formulation sites • 9 global packing sites |
| Data Requirements | Up to 6 stability studies: • 3 batches per strength |
Up to 36 stability studies: • Complex matrix of formulation and packing site • Cost of up to $4.5 million |
| Implementation Globally | At product launch | Up to 5 years from first stability set down |
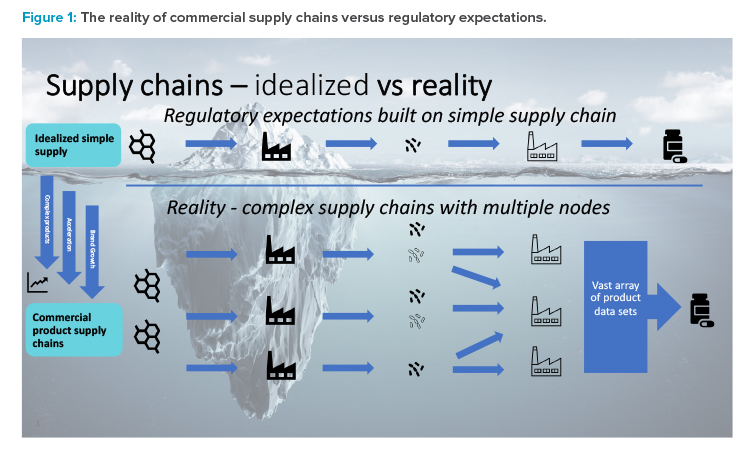
For commercialized medicines, the equivalent development and switch to a more sustainable packaging material is significantly more challenging. This is largely due to the necessary postapproval regulatory action, lack of harmonized supporting data requirements, and varying approval times observed in different markets. For established medicines supplied globally, the difficulty is further increased by the complexity of supply chains and the impact this has on regulatory data requirements. Figure 1 provides a graphical representation of the situation, where regulatory expectations are built on an idealized simple linear supply chain, i.e., single API site, single formulation, and packing sites.
The reality is that commercial supply chains are becoming increasingly more complex with multiple nodes at every stage, driven by brand growth, the need to accelerate the supply of medicines to patients, and new products becoming more complicated due to the need for specialized equipment for certain unit operations and this being available at specific sites only.
As shown by this sustainable packaging material example, the result of these complex commercial supply chains is that the data requirements to support postapproval changes in the commercial space are vastly increased. Table 1 provides an example of a global product with multiple strengths and packing sites. Due to differing market regulatory requirements—such as packing site-
specific stability studies and registration samples—the volume of data required to support changes such as these is large, demands significant investment to generate the stability data, and takes a considerable amount of time to implement due to both data generation and lengthy regulatory variation procedures.
There is a case for regulatory authorities worldwide to recognize scientific approaches and base their required data package on scientific and technical rationale rather than a request to simply produce data. For example, when introducing an alternative packaging material that is demonstrated to be equivalent or superior, there is no scientific need for additional site-specific stability data to be generated from an established packing site.
The impact of this lack of regulatory harmonization for both regulatory procedural timeframes and data requirements vastly increases the complexity of introducing sustainability-driven improvements to commercial medicines, which creates a barrier for industry. Global harmonization of approval times and requirements, such as a single data package applicable to all markets, would facilitate faster implementation, making changes such as this more achievable for industry to implement.
As stated previously, the onus is on pharmaceutical manufacturers to develop sustainable packaging solutions during product development ready for commercial launch. For commercialized products, however, there is a need for authorities to harmonize regulatory procedures and data requirements to make switching to a more sustainable packaging material a viable and attractive option for pharmaceutical manufacturers. This would reduce the cost and time investment by eradicating unnecessary data requirements based on sound scientific reasoning, which in turn would facilitate faster implementation.
New EU-wide rules were proposed in November 20225 for recyclability requirements for all packaging. According to the proposal, all packaging shall be designed for recycling by 1 January 2030 and be recycled at scale by 1 January 2035. However, exemptions are proposed until 1 January 2035 for immediate packaging (immediately in contact with the medicinal product) for medicinal products for human use. The proposal includes an exclusion from the obligation of a minimum recycled content in plastic packaging for immediate packaging, and for outer packaging in cases where it has to comply with specific requirements to preserve the quality of the medicinal product. The exclusion is justified with human health protection and to avoid any risk to the security of supply and to the safety of medicines.
Case Study Two: Shelf Life Extensions across the Life Cycle
The concept of shelf life extensions is applied differently across the life cycle. Why do submissions require prior approval for commercial products in many markets, but the same markets need no submissions at all for clinical products? Global harmonization with risk-based approaches is required. Does real-time stability data always need to be reviewed by the health authorities or would company internal assessment be appropriate in some situations?
Longer shelf life would be considered for a number of sustainability-driven reasons, such as to:
- Reduce waste and unnecessary product destruction due to short shelf life by increasing the expiry date for new products without health authority prior approval, provided that quality, safety, and efficacy of the drug product can be confirmed by internal company assessment
- Lower carbon dioxide emissions from transportation, made possible by decreasing the number of in-market replenishments as larger quantities of products could be sent to markets in a single shipment; this is especially relevant for the markets that require 75% remaining shelf life for customs clearance
Clinical Supply
Development of a Formulation
The drug substance to be investigated in a clinical development program must be administered as a formulation. This formulation will change during the clinical development program. In early clinical phase (phase 1 and 2A), a simple but not patient-friendly formulation is used. An example is an oral solution or suspension that is stored frozen. The formulation must be thawed, diluted, and poured into a dosing cup before being administering to a participant in a clinical trial. Administration is usually performed at a hospital and supported by a pharmacy at the hospital.
For late clinical phase (phase 2B and 3), a patient-friendly but complex formulation is developed. An example is an oral modified release tablet with a functional coating. The tablets are packed in primary and secondary packaging by the sponsor of the clinical trial. Administration is usually performed at home and there is no involvement of a pharmacy.
Shelf Life
For a new formulation—for example, a tablet—the shelf life and storage conditions should be defined based on the stability profile of the drug substance and the available stability data for the drug product. If there is a limited amount of stability data obtained, then the shelf life will be short.
As a development project progresses from early to late clinical phase and switches formulations, the short shelf life for the new formulation becomes problematic. There is insufficient time to generate stability data to ensure a shelf life suitable of meeting the duration of late-phase clinical trials. There are two options to solve this.
- Extend the shelf life for already manufactured supply as more stability data becomes available. This needs to be addressed in the initial clinical trial application and for some countries as amendments to the approved clinical trial application.
- Waste the already manufactured supply and manufacture new supply. This conflicts with sustainability regarding using natural resources in the best way.
Considering sustainability, wasting current supply and manufacturing new product should be avoided as a priority, especially when stability data demonstrates the existing product continues to be safe to use. Opportunities to facilitate this exist, such as provision of a shelf life extension plan as part of the initial clinical trial application, which allows the shelf life to be extended without a prior approval submission in the majority of markets, as shown in Table 2.
| Notification Before Implementation | Notification After Implementation | No Notification | No Shelf Life Defined |
|---|---|---|---|
| Brazil | Canada | Russia | Argentina |
| China | Taiwan | Israel | |
| European Union | Japan | ||
| Norway | Mexico | ||
| UK | South Africa | ||
| US |
| Period (Months) | ||||||
|---|---|---|---|---|---|---|
| Available Stability DataProposed |
6 | 9 | 12 | 18 | 24 | 36 |
| Proposed Shelf Life |
18 | 21 | 24 | 30 | 36 | 36 |
In the EU, extrapolation may be used if stability studies are conducted in parallel to and throughout the duration of the clinical studies.6 Extrapolation is the practice of using a known data set to infer information about future data.7
Any proposal for a future shelf life extension without a substantial modification submission should be stated in the clinical trial application. A stability protocol covering the maximum planned shelf life, statement to confirm reporting to the competent authority of any significant negative trend in results, and the shelf life extension plan should be provided. An example of a shelf life extension plan is shown in Table 3.
This demonstrates that there are countries that encourage faster implementation of sustainability initiatives for clinical supply (i.e., extending the shelf life for already manufactured supply) by using risk-based scientific approaches (i.e., the shelf life extension plan). If more markets took this approach, sustainability would be improved.
Commercial Supply
Launch of a New Product
At the point of submission of a new marketing application, the minimum allowable amount of stability data is 12 months at the long-term storage condition and 6 months at accelerated conditions for batches representative of the commercial product.8 In an ideal situation, the commercial formulation is the same as used in phase 3 clinical trials and it may be possible during the review period to provide additional real-time stability data to justify a longer shelf life upon approval. However, this granted shelf life is not always sufficient to meet the needs of the supply chain to ensure continued supply to patients, and as more real-time data becomes available the shelf life is increased by postapproval regulatory updates.
Packing Sites
For a product launched globally it’s not unusual to use several packing sites at different geographical locations. Market differences in regulatory data requirements, such as packing-site-specific stability data, has implications on the data required for the shelf life because stability data is required to be generated for each packing site in the supply chain (as described in case study one), therefore significantly increasing the cost and quantity of data needed to be generated. If markets were to harmonize requirements and recognize scientific and technical rationale, the need to generate additional data could be avoided.
Extending the Shelf Life for Commercial Products
Similar to clinical products, data from stability studies must demonstrate that the approved end-of-shelf-life specifications are still met in order to extend the shelf life for a commercial product. Extrapolation of existing data can also be employed for commercial products, although the majority of markets do not recognize this and insist on the provision of real-time data. The major difference for commercial products is that regulatory submissions are required to be approved prior to implementation of the new shelf life in the vast majority of markets. Egal and Lombardi9 summarize the current regulatory reporting categories for pharmaceutical products shelf life extension in ICH, PIC/S, and WHO member countries. In only 3 out of 63 countries it is allowable to implement a shelf life extension before informing the health authority.
Similar to case study one, it is observed there is no global regulatory harmonization concerning both data requirements and regulatory procedure type. The need for pack-site-specific stability data shows no global regulatory consistency in recognition of scientific approaches, all of which again demonstrates how this acts as a barrier to industry in implementing sustainability changes such as this.
In this case study, it is also evident there is no harmonization between the clinical and commercial regulatory environments for the same market for what should be a relatively simple change based on real-time stability data. If the commercial regulations were to adopt an approach similar to that used for the clinical products, implementation of sustainability changes such as these would be accelerated.
A pharmaceutical quality system (PQS) is a management system used to direct and control a pharmaceutical company with regard to quality. ICH Q1010 describes a model for an effective PQS that is based on International Standards Organization (ISO) quality concepts, includes applicable Good Manufacturing Practice (GMP) regulations and complements ICH Q8 (Pharmaceutical Development) and ICH Q9 (Quality Risk Management).11, 12 A PQS can be implemented throughout the product life cycle and should facilitate innovation and continual improvement.
For postapproval changes, such as shelf life extensions, where data is generated to prove suitability of the proposed change, it should be possible for companies to manage the implementation within the PQS and not have to seek prior approval from regulatory agencies. This could be applied to both the clinical and commercial environments and would facilitate a faster implementation of sustainability-driven benefits. Reference is made to the paper by Egal and Lombardi,9 who raise the question on management of postapproval changes such as this, via PQS only.
Conclusion
Will the current regulations be fit for a sustainable future? To enable changes across the industry, sustainability should be included alongside quality, efficacy, and safety when assessing medicines. Two revisions in how postapproval changes are handled could significantly enable sustainability changes, provided that they are combined with an effective PQS:
- For more sustainable pack types, data requirements should be changed to remove the need for packing site-specific data.
- To extend shelf life, regulatory procedures should be changed to allow notification after implementation.
However, inclusion of sustainability as a regulatory requirement would take legislative action and would not be solely determined by regulators in many countries. This could mean that it would take many years to implement.
The two case studies presented provide the general themes that can be applied to the implementation of any sustainability driver within the pharmaceutical industry for which there is regulatory impact. As industry strives to develop novel and sustainable medicines to meet future patient needs and looks to implement as quickly as possible the reduction in environmental impact of the marketed products, help is required from global regulators in two main areas: harmonization and risk.
Regulatory applications are consistently finding divergence in the interpretation of ICH guidelines by regulators from different countries.13 This divergence becomes a disincentive to improvements and has even caused temporary drug shortages in some markets. When coupled with the differing market data requirements to support regulatory changes, inconsistent approaches to use of scientific rationale, and varying regulatory procedures and timelines, the barriers to industry are high in terms of data generation, cost, time, and complexity of implementation.
Reliance procedures do exist and are used in certain circumstances, but these are not applied consistently or globally. Owing to this global regulatory complexity, individual postapproval changes often take years for full worldwide approval, which reduces the impact of the sustainability improvements they can offer. Current regulatory mechanisms and guidance for these postapproval changes do not consider the company’s latest product and process knowledge when determining the type of filing required to implement the change. The application of ICH Q9 (Quality Risk Management), or the effectiveness of a company’s PQS to manage a postapproval change is not considered during the assessment of a change.9, 14
Application of ICH Q124 could facilitate the introduction of changes to support sustainability. ICH Q12 provides a framework to facilitate the management of postapproval CMC changes in a more predictable and efficient manner. The Post-Approval Change Management Protocol (PACMP) is a regulatory tool that provides predictability regarding the information required to support a CMC change and the type of regulatory submission based on prior agreement between the marketing authorization holder and regulatory authority.
Such a mechanism enables planning and implementation of future changes to established conditions (ECs) in an efficient and predictable manner. The PACMP may be submitted with the original marketing authorization application or subsequently as a standalone submission and can be proposed independent of any prior identification of ECs. The PACMP requires approval by the regulatory authority, and the conditions and acceptance criteria outlined in the protocol must be met and results communicated to the regulatory authority in the manner previously agreed, in order to implement the change(s).
According to the information on the homepage of ICH in January 2023, ICH Q12 has been implemented in the US and Japan but implementation has not been completed in Brazil, Mexico, European Union, Singapore, Canada, Korea, UK, China, Saudi Arabia, Switzerland, Chinese Taipei, or Turkey.
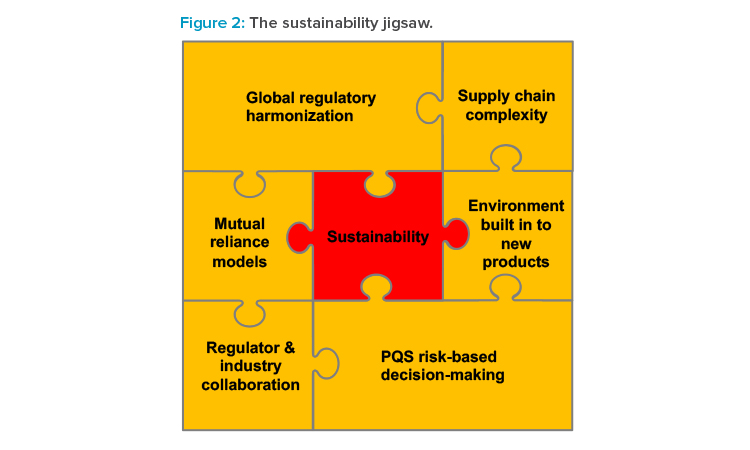
Sustainability is at the heart of a complex regulatory jigsaw (see Figure 2) connected to the themes presented in this article. The current regulatory frameworks for manufacturing changes to medicines have evolved nationally and regionally and are built on patient safety considerations and safety disasters from the past.
These frameworks are not globally harmonized and do not consider future risks such as the environment. Should authorities be doing more to drive sustainability into medicines by building this into regulatory expectations? Is a modern framework required that includes consideration for the environment, should it be quality, safety, efficacy, and sustainability? Should authorities characterize the carbon footprint and/or environmental impact of an approved product? Should there be an expectation that pharmaceutical manufacturers produce an action plan for reducing the carbon footprint of their medicines?
From the discussions presented previously, one thing remains clear: to drive sustainability forward, industry and regulators require implementation of risk-based approaches as described in ICH Q9, Q10, and Q12. Without the necessary support from global regulators, can industry really deliver on the ICH Q10 expectations of innovation and continual improvement, and utilize this to improve the sustainability profiles of the medical products which impact the lives of so many?




