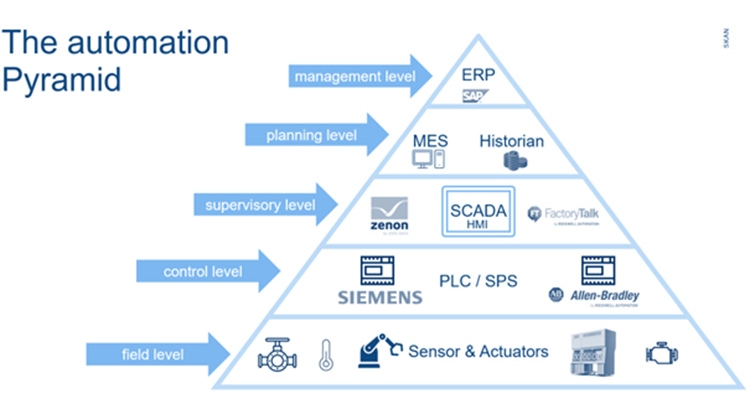
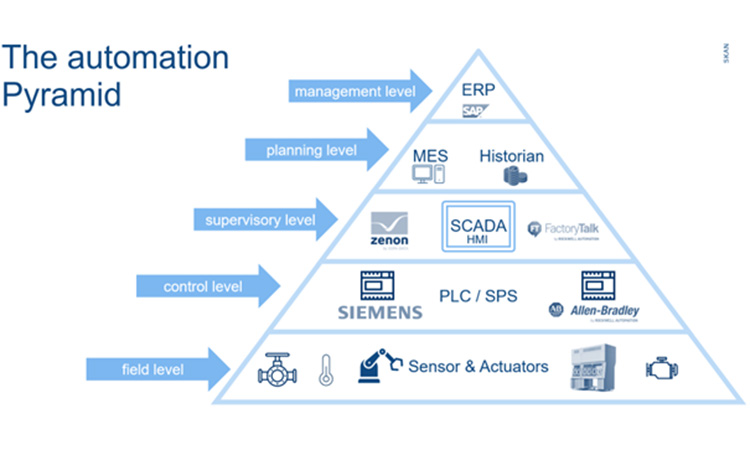
4. Annex 1 - Challenges and Contribution of CMOs
There are several challenges that can arise during a product transfer to a CMO. Some of these challenges include for example regulatory compliance, quality control and effective communication between the original manufacturer and the CMO.
Product transfer to a CMO is typically used to take advantage of cost savings, access specialized expertise or equipment, or increase manufacturing capacity. The transfer process is complex and involves several steps, including the selection of the appropriate CMO, the preparation and transfer of documentation, and the validation of the manufacturing process to ensure that the product is manufactured consistently and complies with regulatory requirements, such as those set by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
The final step of full-scale manufacturing is also critical, requiring compliance with cGMP, maintenance of quality systems, and the oversight of the process to ensure that the product is manufactured to the required standards.
As the pharmaceutical industry continues to evolve, the use of CMOs for product transfer is likely to become increasingly common, as companies seek to streamline their operations and increase their competitiveness in the marketplace. By partnering with a CMO, pharmaceutical companies can focus on their core competencies, such as research and development, marketing, and sales, while leaving the manufacturing and regulatory aspects to the CMO. This can allow pharmaceutical companies to bring their products to market faster and more efficiently.
This part of the track will include the following segments:
- Pharma – a 40-year globalization journey
- Is re-shoring of APIs and finished dosage forms to Europe still possible?
- CMO as a platform for geographic flexibility
It’s exciting to see the industry's progress and to bring together experts to share knowledge and insights on these important topics. By fostering collaboration and knowledge-sharing, we can continue to drive innovation and improvement in the pharmaceutical industry.
As emerging leaders (Dany Shami and Natalia Vtyurina) at ISPE we are very excited to be able to support Richard Denk and Amjad Wahbeh with Track 2 – Annex 1. We are especially looking forward to spending the 3 days during the conference in the beautiful city of Amsterdam. Our goal is to gain new knowledge and connect with different leaders from various companies across Europe.
We are confident that the conference will be a great success and hope that you will join us for this exciting event. We look forward to welcoming you to the conference!
Learn More & Register