ICH Q9 (R1): The Next Frontier

A one-day seminar titled ICH Q9(R1): The Next Frontier was hosted by the Pharmaceutical Regulatory Science Team (PRST) at the Technological University Dublin (TU Dublin) on 1 June 2023. Professor Anne Greene of TU Dublin and Marty Lipa, Merck & Co., co-chaired the seminar. The ISPE Ireland Affiliate was a co-sponsor. The objective of this event was to explore knowledge application in three key areas:
- Advancing the effectiveness of quality risk management (QRM) & risk-based decision making
- Enabling innovation in the pharmaceutical workplace of the future
- Supporting digital transformation
This blog summarizes those parts of the seminar which covered the importance of knowledge and its use in quality risk management exercises.
The seminar was organized with opening keynote and plenary presentations followed by three tracks, one for each of the above topics with closing plenary sessions.
The first keynote presentation, “Smart Missions that Enable Innovation in High-risk Environments,” was delivered by Dr. Ed Hoffman, a senior lecturer at Columbia University who retired from NASA as a Senior Executive. He was the first NASA Chief Knowledge Officer and founder of the NASA Project Leadership Academy. Following the Columbia Shuttle failure, he led the team that designed the Strategic Management and Governance Handbook. His outstanding presentation provided important insights regarding learning, and how knowledge is acquired and applied. His work at NASA was extremely important to the organization since NASA almost went out of business in the 2000s due to risk avoidance. Aerospace is healthier today at NASA and SpaceX by balancing risk and innovation. Key learnings were to focus on the teams and the people therein. Clearly, teams should have missions stressing the aims and values of the company, organization, or team, however, “A smart mission recognizes that few things go as planned, and that both learning and unlearning are essential… above all, it values people…” (Hoffman, Kohut, and Prusak, The Smart Mission, MIT Press 2022). Hoffman described the six principles of the Smart Mission integrated systems model, which are Culture, Teaming, Learning, Stories, Global Collaboration and Knowledge. The focus should be on the team and its people:
- Consider your current project team. Which of the six principles are most critical currently? Why?
- What behaviors are you practicing to promote the principle?
An aim is to lower the cost of failure since more planning DOES NOT reduce the chance of failing a new venture. A better approach is the use of more, faster, lower-cost experiments, with more attention paid to lessons that come from all experiments, including those (the majority) that fail. Try to understand what you do not know. In summary, relationships, trust, and culture are extremely important such that teams should be deliberate about how to work together.
Dr. Emma Ramnarine presented the second keynote titled “Balancing Quality & Innovation in Pharmaceutical Manufacturing.” Ramnarine recently joined Boehringer Ingelheim as Head of Product Management & Development Operations for US Operations. Prior to this she was at Genentech Roche for 17 years in various global roles in Product Development, Quality, QC and Analytical Sciences.
Ramnarine commenced by explaining that ICH Q10 Pharmaceutical Quality System was the basis of patient-centric innovation with quality risk management and knowledge management as two important enablers. She used the current problem of drug shortages as an example of a patient-centric problem for which one contributing factor is the issue of the protracted length of time taken by regulatory authorities globally to approve post approval changes (PACs). Many of these changes are necessary to support continual improvement of manufacturing processes to assure continued supply to patients. Ramnarine displayed data produced by the industry One-Voice-of-Quality (1VQ) Initiative of which she is a co-leader, showing:
- 125,886 data points from 16 of the top 25 pharma companies for 2019-2021
- 1 of 156 countries met the WHO recommendation of no PAC approval exceeding 6 months
- 1 in 5 countries take longer than 6 months for the majority of PACs
These long, variable, and uncertain timescales give great complexity and cost in supply chain management regarding which batches made by which process can be supplied to the various markets.
Ramnarine explained that drug shortages are a complex multi-stakeholder problem involving competent authorities, industry players and point of care deliverers. She concluded that solutions require collaboration, science- and risk-based decision making with stakeholders focused on patient-centric solutions.
Dr. Kevin O’Donnell of HPRA, ICH Q9(R1) Expert Working Group (EWG) rapporteur, discussed the background to the review of ICH Q9(R1) and gave some personal thoughts about implementing the new guidance. The original ICH Q9 guidance was issued by ICH in November 2005, however, it was recognized in the ICH Q9(R1) Concept Paper that the benefits of QRM, as envisaged by ICH Q9, have not yet been fully realized. A targeted revision started in December 2020 with the revision finalized in January 2023, which will be supported by training materials in summer 2023. The revision concerned 6 specific topics:
- Subjectivity in QRM
- Product availability risks
- Formality in QRM
- Risk-Based Decision Making (RBDM)
- Risk review
- Hazard identification
High levels of subjectivity are not in line with first principle of Q9: “The evaluation of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient.” Subjectivity can relate to many different things:
- Differences in how hazards, risks and harms are perceived by different stakeholders and how risks are assessed
- Teamworking influences and human heuristics
- The risk scoring methods some risk assessment tool use
While subjectivity cannot be eliminated from QRM activities, it may be controlled using well recognized strategies, including addressing bias and behavioral factors.
ICH Q9 already addressed product availability risks, as its definition of harm includes damage “from a loss of product availability.” There is an increased emphasis in ICH Q9 (R1) on managing product availability risks related to manufacturing problems/issues.
ICH Q9 states: “The level of effort, formality and documentation of the quality risk management process should be commensurate with the level of risk.” A lack of understanding of this has led to confusion and uncertainty within the industry and among regulators regarding what this means in practice. The revised version of Q9 seeks to clarify what formality in QRM means and the factors that might be considered when determining how much formality to apply to a given QRM activity. It also emphasizes how there is flexibility in how much formality may be applied.
While ICH Q9 referred to decision making, there was a lack of clarity on what good risk-based decision-making is, and how it might be achieved. The Q9 revision seeks to provide clarity in this area and addresses the expected benefits of investing in risk-based decision-making activities.
The Q9 revision and the associated training material will provide additional clarity on the expectations relating to maintaining risk management outcomes and on the implementation of risk reviews.
Finally, in the diagram of a typical quality risk management process in Q9(R1) terminology has been corrected from “Risk Identification” to “Hazard Identification.”
Although there are many ways to implement the revised guideline, O’Donnell described one potential way moving from familiarization to understanding the links between knowledge and risk then reviewing how formality and subjectivity could be improved. Finally, a team could introduce more structured risk-based decision making and how to ensure risk reviews are embedded in a QRM processes. O’Donnell provided some examples of how Q9 revision could be applied in several areas of work at HPRA in GMP inspections, recall decision making and in the medicines shortages group.
Dr. Anders Vinther of Kronos Bio provided a senior quality leader’s view on how such a leader could make an impact at business decision making level and make a quality organization add financial value. Great quality leaders constantly focus on patients as well as being great business and people leaders. He stressed a theme for people to learn from quality thinkers e.g., Juran, Deming, Shewart, Crosby and Ishikawa, and to learn from our environment and experiences to develop the knowledge to make difficult risk-based decisions.
In the QRM and RBDM track, Michael Schousboe, Process Manager for Quality Risk Management in Novo Nordisk and a member for EFPIA on the ICH Q9(R1) EWG presented “Risk Based Decision Making – using knowledge of risk throughout the lifecycle.” He explained that highly structured approaches to the QRM process and decision making should be applied when the situation is of high importance with high uncertainty and complexity. Less structured approaches could be used when there is existing knowledge, and uncertainty and complexity are lower. At the lowest level of formality SOPs and policies could be in place based on historical risk exercises and typically are used when uncertainty and complexity are low. Schousboe exemplified less structured, pre-agreed decision-making approaches applied to warehouse validation and to calibration intervals. In both cases, for different possible scenarios, a team constructed pre-assigned risk-based outcomes. Critical to establishment and work of the team clarity was needed on:
- Scope; what decision is about and expected outcomes
- Team experience and structure in terms of knowledge, business understanding and behavioral factors
- Information needed
- Business logic
Valerie Mulholland, a senior consultant at GMP Services, Ireland presented her PhD work at the Pharmaceutical Regulatory Science Team (PRST), “Improving RBDM Effectiveness.” Mulholland described the range of decision complexity from “Tame Problems” characterized by the problem statement can be articulated (e.g., equipment upgrade), “Messy Problems” where the problem statement is hard to characterize (e.g., facility contamination), and “Wicked Problems” where the problem statement is hard to articulate (e.g., drug shortages).
Mulholland had reviewed the literature in other industries e.g. nuclear, aviation and proposed a RBDM process which considered 21 critical attributes categorized by:
- A robust QRM process
- Use of the best available knowledge
- Application of good governance and consideration of human factors
The critical attributes were chosen if they appeared in greater than three of the guidances reviewed and are given in the figures below.
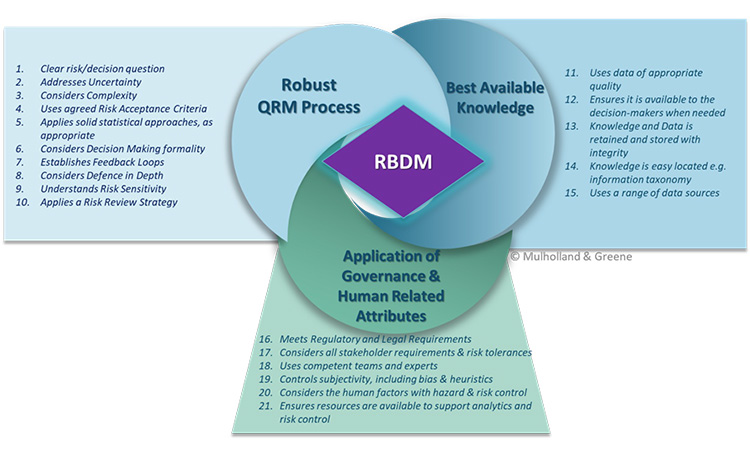
Examining the process of decision making in complex situations of high importance, uncertainty, and complexity, Mulholland proposed a structured approach where at key points in the QRM process a review is conducted assessing:
- Whether there is adequate information to progress OR whether more knowledge is needed
- Whether there are assumptions or uncertainties that require visibility in the next phase
- Whether decisions at each point have been documented appropriately for future reference.
Mulholland concluded by summarizing the significant benefits of a key decision review points process.
Jean-Louis Robert, former chair of the EMA CHMP/CVMP Quality Working Party topic lead for ICH Q12, Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management presented the “Long Road to ICH Q12”. Robert explained that ICH started on scientific regulatory guidelines such as stability and impurities. An important ICH Quality Statement in 2003 - “Develop a harmonized pharmaceutical quality system applicable across the lifecycle of the product emphasizing an integrated approach to quality risk management and science” - led to the need for ICH guidelines Q8, Pharmaceutical Development, Q9 Quality Risk Management, and Q10 Pharmaceutical Quality System. Q8 described the science- and risk-based approach to drug product development activities with Q10 applying to drug substances and drug products throughout the lifecycle. Subsequently, the science- and risk-based approach was described for drug substances in Q11. Since the benefits of ICH Q8 and 11 were hard to realize, a guideline, again based on science and risk has been developed for post approval change management, ICH Q12. The basis of the risk part of these approaches is given in Q9. Robert proposed that the approach given by Q12 should lead to advantages for sponsors compared to the existing Variations system in the European Union.
In conclusion, this excellent seminar brought together a great number of thought leaders in the fields of generation of knowledge and application of quality risk management to development, manufacture, and supply of pharmaceutical products. The presentations and discussion in panel sessions highlighted the importance of understanding how knowledge is generated and applied to reduce risk. Revision of ICH Q9 provides guidance for organizations to give more thought regarding their risk management processes to reduce subjectivity, consider formality, and have structured risk decision making.



