Conceptualizing a Streamlined Performance Assessment – The St.Gallen APQ Benchmarking Tool
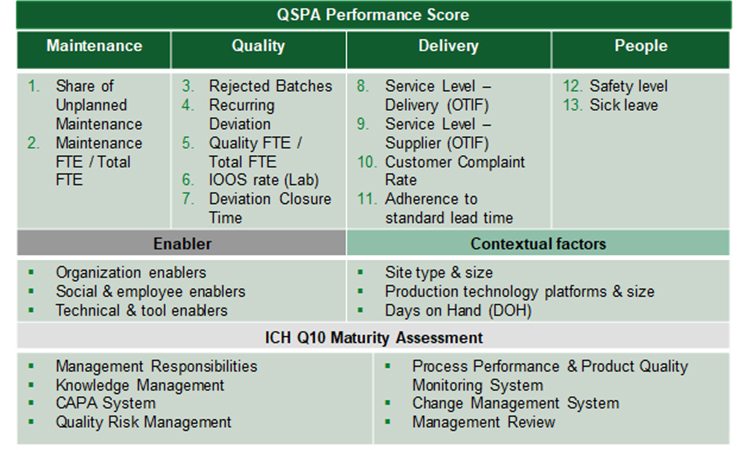
The ISPE APQ Assess, Aspire, Act and Advance Framework will be complemented with an OPEX Benchmarking and ICH Q10 maturity assessment tool specifically developed for use with the APQ program by the University of St.Gallen, Switzerland. Used in conjunction with the APQ self-assessment tools for quality management maturity, the pre- and post- benchmarking activities offer objective evidence of performance improvement to support ongoing investment of time and resources along the APQ framework. The effectiveness and impact of the improvements can be evaluated and quantified to derive priorities for next improvement opportunities. This article provides the scientific background of the assessment tool