iSpeak Blog
iSpeak Blog
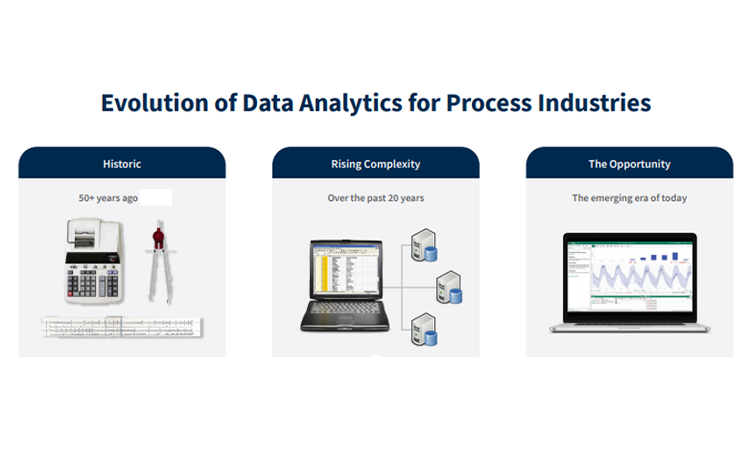
ISPE’s Q12 Implementation team, a working group under the auspices of ISPE's Product Quality Lifecycle Implementation (PQLI)® committee, continued their series of training events with a well-attended course delivered to Singapore’s Health Sciences Authority (HSA) in November 2024.