Process & Product Contact Surfaces in Bioprocessing

As part of their quality risk management (QRM) programs, many biopharmaceutical manufacturers have made deliberate efforts to classify systems, equipment, and components by their potential to affect product quality. In assessing these risks, it is intuitive to focus on component surfaces that come into direct contact with process fluid streams and that are integral to the biopharmaceutical manufacturing process. A great deal of effort goes into the specification and verification of these surfaces, particularly those that come into direct contact with active pharmaceutical ingredients (API) or API intermediates. For example:
- Construction materials are specified and documented for traceability to be nonleachable, nonadsorptive, and nonadditive.
- Surface finish and treatment are specified to facilitate cleaning (e.g., alloys are electropolished to a mirror finish and/or chemically passivated to limit rouge).
- Surfaces are cleaned and rinsed to a point at which rinse-water quality attributes meet those established for water for injection (WFI).
- Surfaces are often sterilized to achieve a sterility assurance level (SAL) of 10–6 (six-log reduction).
The resources required to design, fabricate, install, verify, and maintain these surfaces are substantial, but the commitment has a commensurate benefit to product quality. It is therefore imperative to have standard definitions by which these select surfaces can be classified in a QRM program.
This article presents definitions for process contact surfaces, product contact surfaces, nonprocess contact surfaces, and product containing surfaces. The American Society of Mechanical Engineers (ASME) Bioprocess Equipment (BPE) standard, beginning with the 2014 edition, provides the most comprehensive and useful definitions for process contact surfaces and product contact surfaces and the implications of distinguishing between them.
There are a number of ways to determine appropriate definitions, but many fall short. This article presents three approaches to demonstrate the clarity and usefulness of the ASME BPE’s approach in comparison to other approaches. Additional definitions for nonprocess contact surfaces and product-containing surfaces are proposed for completeness, as well. This article also discusses the importance and implications of formulating such distinct definitions.
Definition Paradigms
There are several approaches to defining process and product contact surfaces. Three methods are examined critically here.
Approach 1: Literal definitions
One way to define the difference between process contact surfaces and product contact surfaces is by a literal definition.
Process contact surfaces: Surfaces of components that may be exposed to process fluids/solids and have the potential to transfer material into the product or onto product contact surfaces.
Product contact surfaces: As a subset of process contact surfaces that may be exposed to product or product constituents under design operating conditions, and from which material may drain, drop, drip, or be drawn into the product.
The phrase “may be exposed” is intended to include surfaces that are not normally in direct contact with the process fluid/solid, but are exposed to the process. For example, portions of a clean-in-place (CIP) rinse tank headspace may not come in direct contact with process fluids, but they are exposed to them. If a contamination were to develop on these surfaces, there is nothing to prevent that contamination from being transferred to product contact surfaces as part of the cleaning process.
The phrase “under design operating conditions” is intended to exclude the failure of components or the use of components for conditions outside of their design basis. The term “product constituents” is intended to extend the definition to API intermediates. The terms “drain, drop, drip, or be drawn” are intended to extend the definition to surfaces that may inadvertently come into direct contact with the product. They are inspired by the definitions in 3-A Sanitary Standards, Inc., (3A)1 and European Hygienic Engineering & Design Group*—(EHEDG).2
3A definition of product contact surfaces: “All surfaces which are exposed to the product and surfaces from which splashed product, liquids, or material may drain, drop, diffuse {where applicable}, or be drawn into the product or onto product contact surfaces.”
EHEDG definition of product contact surfaces: “All surfaces of the machine that intentionally or unintentionally come in contact with the product, or from which product or condensate may drain, drop, or be drawn into the product or container, including surfaces (e.g., unsterilized packs) that may indirectly cross-contaminate product contact surfaces or containers.”
The literal definition approach falls short because an example can always be found that eludes the intent. Drain systems, for example, are exposed to product, but they are generally not considered critical to product quality. In addition, each biopharmaceutical manufacturer is able to define “product” differently, adding further complications.
* Both EHEDG and 3A deal with the same or similar equipment and processes as that used in biopharmaceutical manufacturing and are credible sources for seeking a definition for product/process contact relating to hygienic design.
Approach 2: Surface exposure and conditions
A second approach is to define process and product contact surfaces both by exposure and by specified treatment. A fundamental reason for trying to make the distinction between product and process contact surfaces is to establish rationales for how they should be treated (i.e., cleaned, sanitized, sterilized, and/or isolated). Definitions on the basis of treatment would lead to the analyses and associated definitions shown in Table A.
| Conditions | Process Contact Surface | Product Contact Surface |
|---|---|---|
| Exposed to product | No | Yes |
| Exposed to process fluids/solids | Yes | Yes |
| Cleaning required for bioburden reduction | No | Yes |
| Cleaning required to reduce the potential for cross-contamination | No | Yes |
| Sanitization or sterilization required | Yes, if required by the process | Yes, if required by the process |
Process contact surfaces: Surfaces of components that by design are not directly exposed to product and do not require cleaning of product residue (e.g., clean steam, compendial water, once-through CIP), but may require sanitization or sterilization for bioburden control.
Product contact surfaces: Surfaces that are exposed to final product or product intermediates, and require cleaning to reduce bioburden and the potential for cross-contamination (e.g., bioreactor vessels and nondedicated transfer lines), and may require sanitization or sterilization for bioburden control.
These definitions also fall short in that some bioprocessing components are excluded. Single-use components, for example, could have product contact surfaces, may be required to be sterile, and yet may not be required to be cleaned or sterilized after product exposure.
Approach 3: Establishing requirements
A more comprehensive approach would be to establish up front all the requirements with which the definitions would be required to comply. This would ensure that the resulting definitions apply to the surfaces of the intended components/systems.
Process contact surface requirements must:
- Be applicable to overall use for hygienic service
- Be applicable to single-use technology
- Include solids handling surfaces
- Include the potential for contact with process materials (indirect contact or exposure)
- Include contact with raw materials, in-process materials, APIs, clean utilities, and CIP supply
- Exclude drain systems
- Exclude abnormal upset events outside the scope of the design
Product contact surface requirements must:
- Be a subset of process contact
- Be applicable to single-use technology
- Allow the affected organization to define “product”
- Include the potential for contact with “product” (indirect contact or exposure)
- Include process surfaces that contact “product” and/or have the potential for crossover contamination
- Exclude drain systems
- Exclude abnormal, upset events outside the scope of the design
The resulting definitions, as first adopted by the ASME BPE Standard in 2014: 3
Process contact surface: Surfaces that, under design operating conditions, are in contact with or have the potential to contact raw materials, in-process materials, APIs, clean utilities (e.g., WFI, CIP, pure steam, process gases), or components (e.g., stoppers), and where there is a potential for the surface to affect product safety, quality, identity, strength, or purity.
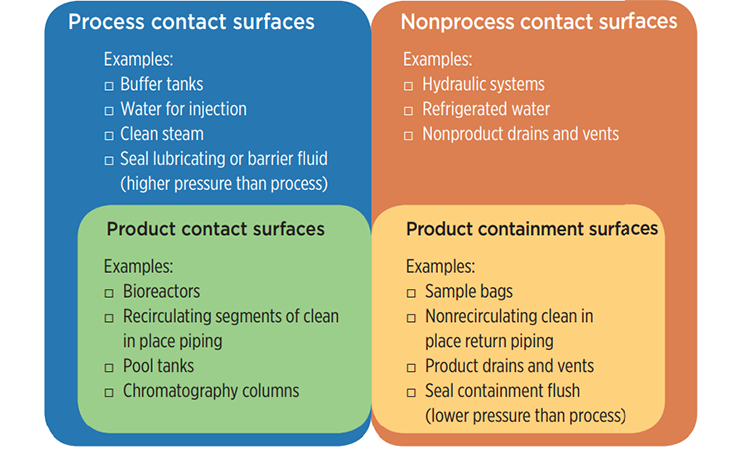
Product contact surface: Process contact surfaces that are in contact with or have the potential to contact product, where product is defined by the owner/user. Examples of product contact surfaces may include the interior surfaces of bioreactors, transfer tubing, chromatography columns, vessels, and recirculating segments of CIP systems.
The ASME BPE standard is widely accepted for best practices in hygienic design in the biopharmaceutical industry. Both definitions above can be found in Part GR: “General Requirements” of the standard, and are used throughout to set design requirements for components used in bioprocessing.
As product contact surfaces are a subset of process contact surfaces, requirements for process contact surfaces are applicable to product contact surfaces. Requirements for product contact surfaces, however, are not necessarily applicable to process contact surfaces. The applicability of these definitions with respect to systems that are common to biopharmaceutical processing is examined in Table B.
The ASME BPE Standard does not define nonprocess contact surfaces, but does imply that if the surfaces do not comply with the definition of process contact surface, it is a nonprocess contact surface. For completeness the following definition is proposed:
Nonprocess contact surface: Surfaces that, under design operating conditions, do not have the potential to affect product safety, quality, identity, strength, or purity.
| Component Surface | Process Contact | Product Contact |
|---|---|---|
| Bioreactor | Yes | Yes |
| Product transfer lines | Yes | Yes |
| Media vessel | Yes | No, unless media is defined as product |
| Chromatography skid | Yes | Yes, but only those portions, which have potential exposure to product as, defined by the organization. |
| Buffer prep vessel | Yes | No, unless buffer is defined as a product by the organization |
| Compendial water generation and distribution | Yes | No, unless compendia water is defined as a product by the organization |
| Process gases pre-final filter | Yes | No |
| Process gasses post-final filter | Yes | Yes, if serving product containing system |
| Once-through CIP | Yes | No |
| Recirculating segments of CIP* | Yes | Yes |
| Stopper processor | Yes | No |
| Clean steam generation and distribution | Yes | No |
| Drain system | No | No |
| Hydronic fluid system | No | No |
| Clean steam condensate system | No | No |
| Plant steam system | No | No |
* There remains some debate on whether recirculating segments of a CIP system should be classified as product contact as no “product” contacts the recirculating segments. However, the potential for cross contamination of product fall under the phrase “… the potential to contact product …” in the product contact definition, and therefore appears in the 2014 ASME-BPE definition.
While nonprocess contact surfaces do not by definition have the potential to affect product quality, there are circumstances in which design requirements for product or process contact surfaces would apply. When there is a concern for worker safety due to exposure to APIs or potent compounds, for example, hygienic design elements may be required to render containment systems clean and free of chemical and biological hazards. For this reason, a specific definition is useful for product containment surfaces.
Product containment surface: Nonprocess contact surfaces that are in contact with, or have the potential to contact potentially harmful materials (e.g., APIs, potent compounds), where there is a potential for human contact with the surface (e.g., operators, maintenance workers, or the public).
The Venn diagram presented in Figure 1 shows examples of systems with process contact surfaces, product contact surfaces, nonprocess contact surfaces, and product containment surfaces.
Definition Implications
The benefits of distinguishing among components with process, product, nonprocess, and product containment contact surfaces can be realized in a number of applications within a QRM program or other biopharmaceutical manufacturing business processes.
- 1European Hygienic Engineering & Design Group. “EHEDG Glossary: Version 2013/12.G03.”http://ehedg.org/uploads/EHEDG_Glossary_E_2013.pdf
- 23-A Sanitary Standards, Inc. “Designing for Cleanability: Lessons from the Food Industry.” US FDA Public Workshop: Reprocessing of Reusable Medical Devices, 8–9 June 2011.
- 3American Society of Mechanical Engineers. Bioprocessing Equipment: ASME BPE-2016
Design specifications
An important implication is the design and design verification of components used in hygienic service. Construction materials, surface finish, and surface treatment of components with process contact surfaces may be specified to a grade commensurate with the intended service, process requirements, and risk to product quality. WFI distribution piping, for example, which has process contact surfaces, may be specified to be mechanically polished and passivated. In comparison, the surface finish of a bioreactor, which has product contact surfaces, may be specified to have an electropolished surface finish.
Risk-based commissioning and qualification
Distinguishing among product, process, and nonprocess contact components does not simply save the cost of over-specification. It can also reduce the cost of installation and operational verification substantially. In a risk-based qualification program, the components can be categorized as product contacting, process contacting, and nonprocess contacting.
The level of verification documentation can vary as commensurate with the level of risk to product quality. For nonprocess contacting components, the verification might be limited to catalog information such as manufacturer and model number, while process contacting components would require verification of materials of construction, material joining, surface finish, etc. On the basis of a risk assessment, operational verification testing might also be justified in treating process contacting components as those that require commission testing with engineering oversight, and limit testing with quality oversight to systems with product contact surfaces.
Cleaning requirements and cleaning validation
Cleaning requirement specifications could be based on component exposure to product, or only to process fluids. If an organization does not consider chromatography buffers to be “product,” for example, then buffer preparation components may be designated as process contacting components. Such a designation would justify the use of compendial water for cleaning and avoid the use of chemicals required for cleaning product contact surfaces, where the focus is on product carry over. For equipment that contains both product and process contact surfaces, maximum allowable carry over limits may be based only on the product contact surfaces, effectively increasing the acceptable bioburden in the cleaning acceptance criteria for that unit operation.
The product contact and process contact definitions are focused on product quality risks, identified as safety, quality, identity, strength, or purity. Waste or vent systems, according to the ASME BPE definitions, would be classified as nonprocess, as they would not present a risk to product quality. When dealing with potent compounds or any materials at or above Biosafety Level 2, however, the concern for safety of operators and maintenance staff could result in applying cleaning requirements commensurate with those associated with product contact surfaces, replacing “product safety, quality, identity, strength, or purity” with “worker safety.”
Steam sanitization vs. steam sterilization
The distinction between process and product contact can used as a basis to determine which system surfaces should be steam sanitized and which should be steam sterilized. Steam sanitization of process contact surfaces might be specified for the reduction of bioburden for hygienic service, even though the component surfaces are not exposed directly to product. Steam sanitization verification or validation could be limited to monitoring of system temperature elements. Steaming of product contact surfaces, however, might require validating that steam sterilization achieves a specified SAL based on the risk to product quality.
Validating steam sterilization of product contact surfaces requires a significant amount of test data to demonstrate sterility. Multiple test runs would be required, as would in situ temperature mapping, biological indicator testing, and steam saturation verification. It might also be necessary to perform significant cycle development work before testing for sterility, as well as post-sterilization testing, such as sterile hold testing.
Supplier auditing and discrepancy evaluation
As part of a risk-based vendor audit program, making a distinction between components with product contact surfaces and components with only process contact surfaces could help limit the number of suppliers considered “critical” for auditing. Further, if a qualified supplier discovers and reports that a component provided to a customer has a quality attribute outside the reported specification, the risk to product quality can be assessed more readily if the component is known to be a part of a system with product contact surfaces, process contact surfaces, or nonprocess contact surfaces.
Technology transfer
When product manufacturing is outsourced to a contract manufacturer or expanded to another site, a risk-based methodology may be employed to limit physical differences in manufacturing equipment, based on the potential to affect product quality. System components with product contact surfaces are more critical than those with only process contact surfaces. Components with product contact surfaces might be required to be identical to the original site to limit the risk of noncomparability. For components with only process contact surfaces, the degree of similitude could be limited to demonstrating an equivalent function. Nonprocess components could have complete design latitude.
Conclusion
All of these business processes benefit from distinguishing process contacting, product contacting, nonprocess contacting, or product containing components. Depending how an organization defines “product,” the fidelity could be refined even further. Product contact could be divided into API product contacting and non-API product contacting. Under this scenario, a bioreactor could be API product contacting, but a media vessel could be non-API product contacting.
Whatever the breakdown, there are clear benefits to crafting definitions for process contact surfaces and product contact surfaces to distinguish between critical and noncritical components. Further, embracing this distinction within an organization’s QRM program demonstrates to regulatory authorities that the manufacturing processes are well understood relative to their impact to product quality.
Using these definitions to understand the risk to product quality will help organizations decide which components should be fabricated under the most stringent design requirements, which equipment should be qualified, which surfaces require cleaning validation, which surfaces require sterilization (and not just sanitization), which spare parts from a supplier are critical, and which design aspects are critical to technology transfer.


