Using Industry Survey Data to Shape Cell Therapy Facility Design

Cell therapies have been used to treat thousands of patients worldwide ever since the CAR T cell medication Kymriah was the first cell therapy approved by the FDA in 2017. Yet significant manufacturing challenges continue to hamper patient access to life-saving cell therapies, particularly the high cost of these treatments. Kymriah can cost as much as $475,000 per dose and an allogeneic cell therapy for metachromatic leukodystrophy (MLD)—which was approved by the UK’s National Health Service—comes with a $3.9 million price tag. Other cell therapies have been removed from the European market because of similarly high prices.1, 2, 1
We gathered insight from more than 300 industry experts through our CRB Horizons: 2022 Life Sciences report4 to inform a vision for cell therapy facility design that can help address this and other challenges. Cell therapies hold incredible promise to treat previously incurable conditions such as cancers and autoimmune disorders. But they come with unique challenges, owing to the personalized nature of the treatments—one patient per treatment for autologous cell therapy. Manufacturing such small batches requires specialized equipment and skilled operators.
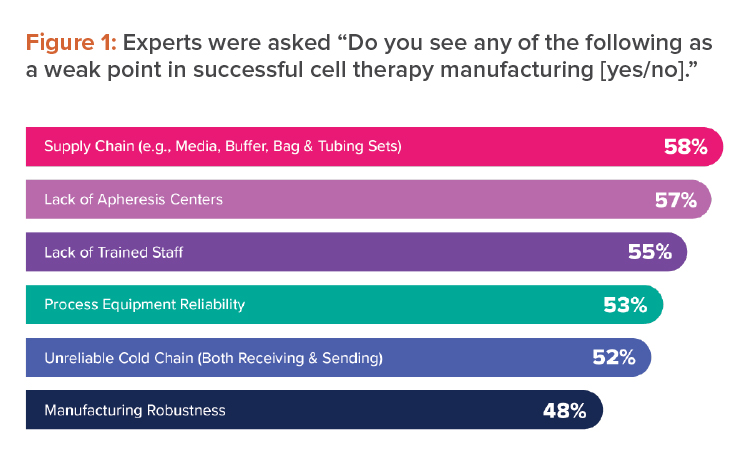
These were among the weak points that industry experts identified when we asked them about successful cell therapy manufacturing for CRB’s Horizons: 2022 Life Sciences report (see Figure 1).4 To broaden patient access to these curative therapies, any solution to the challenges of cell therapy manufacturing must reduce costs.
In this article, we will discuss how designing flexible, commercial-ready facilities is important to address three of the many challenges:
- Attracting and retaining talent
- Lowering overall cost of goods
- Preparing for a smooth technology transfer
Specifically, the solutions include:
- Adopting a platform-based approach to improve scalability
- Improving readiness for a point-of-care model
Together, these will go a long way to alleviating the significant roadblocks to patient access.
Attracting Skilled Staff in a Competitive Environment
The scientists and technicians who work to provide curative cell therapies continue to be among the most important resources in all pharmaceutical manufacturing. Yet when industry experts were asked to list weak points in successful cell therapy manufacturing, 55% chose lack of trained staff (see Figure 1).
This is largely because of the unique technologies and processes used in cell therapy manufacturing. Even for manufacturing operators with experience making traditional biologics, the different skills require significant retraining. The tremendous competition to attract and retain good operators means successful companies will need to differentiate beyond the standard offerings of competitive salary and benefits.
Designing facilities that are vibrant and pleasant and that promote the positive aspects of the science and culture of producing curative therapies can create an environment that skilled operators will want to work in. This is a conservative industry, where tradition and perception have been set, and costs usually eclipse other factors. But when companies pay attention to the aspects of design that affect staff, they can improve facility functionality, increase productivity, and reduce human error. Here we outline some ways to use design to improve worker experience and a facility’s function.
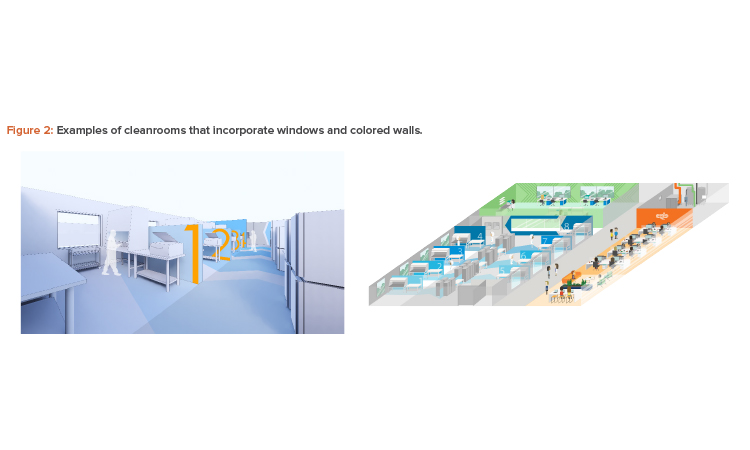
Provide a Vibrant Workplace
Years ago, it was not uncommon to construct a cell culture process facility with prefabricated concrete panels and no windows, which led to a degree of frustration and burnout for employees. Designing a facility in which people are happy to work might mean, instead, ensuring visual connections between rooms and the outside, as well as expanding the traditional color palette.
These are easy ways to improve the way spaces function and the overall working environment (see Figure 2). For example, although white connotes sterility and cleanliness, there are no regulations stating that cleanrooms must be white. Designing an attractive facility could also require investing in a region where real estate and construction costs are higher per square foot—if that location fosters creativity.
Staff will be more productive when they have access to decent amenities, such as a library, an attractive cafeteria/break area, and a natural setting to meet outside. Given the collaborative nature of science, it makes sense to have a pleasant space for people to get together and share their challenges and ideas.
Design for Wellness and Productivity
Cell therapy facilities continue to rely on manual operations, performed by numerous staff who must spend large parts of their workday in gowns, gloves, masks, and safety glasses, often in rooms with little daylight and no exterior views. This can be disorienting and adversely affect physical and mental health. Providing windows to bring in light and sightlines to the outside improves a workspace.
Celebrate the Science and Culture
Creating visual connections between corridors or other spaces and the manufacturing suites can spark excitement about the science. Adding windows to walls within the facility can provide views into the inner workings of the facility for visitors and employees.
Good design blurs the lines between functional spaces to promote collaboration and visual communication. Inclusivity between functional work teams can bring together the PhD scientists and operators with non-manufacturing staff to allow people to learn about the science happening in their facility.
Lowering the Cost of Goods
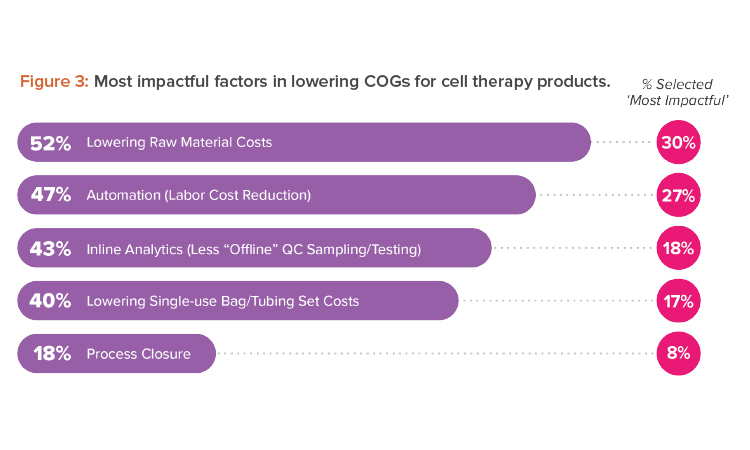
What can life-changing therapies do if we cannot get them to the patients in need? Reining in costs at every opportunity can go a long way to making effective cell therapies more accessible. Among the most impactful factors to lower cost of goods (COGs) that we, as designers, can support are process closure costs and automation (see Figure 3).
The equipment used in research and development labs and for clinical trial production involve manual processes with open connections performed in a Grade A biosafety cabinet with Grade B room background classification. Manual, open manipulations have a greater risk of failure, and high failure rates increase costs. Scaling this approach to meet commercial capacity can have inherent risks that increase with scale.
Design can help COGs through automation and process closure. A thorough analysis of the process will allow a determination of how to reduce risk while procuring, or designing, the appropriate equipment.
The Advantages of Process Automation and Process Closure
We found it surprising that only 18% of survey respondents identified process closure as an impactful strategy to decrease COGs. Cell therapies cannot be sterilized, meaning cell therapy manufacturers use aseptic processing to protect the quality and safety of their products and comply with applicable regulatory requirements. Because there are synergies between automation and process closure, our experience shows that both must be explored to find their full potential.
Process closure is key to scaling cell therapy manufacturing and can result in significant cost savings. The move away from open, manual processing reduces cleanroom classification, minimizes staffing requirements, and lowers the risk of cross-contamination. In addition to the advantages for manufacturing, process closure helps reduce the footprint needed for supporting functions, such as gowning and environmental monitoring. It is best to consider these process and equipment needs early so they align with the company’s manufacturing objectives.
Likewise, system automation is becoming essential for commercial success. Pharma 4.0™ has encouraged the industry to swap manual operations for automated manufacturing technologies. Regulatory agencies want companies to apply the best available technologies and anticipate future developments. Pharma 4.0™ addresses data capture and analysis, as well as inline analytics, aspects that can instill confidence in regulatory agencies regarding the commercialization of these therapies. Data can be collected, analyzed, and applied to adapt and improve processes without extensive disruptions. When the closed processing equipment leads to a lower room classification, and this is combined with the reduction in labor needs through automation, these two factors have the synergistic effect of driving down operating expenses.
Preparing for Smooth Technology Transfers
GMP manufacturing requires the necessary capital, facilities, and trained staff, which may be unavailable to a company with a promising drug candidate. For these reasons, a company is likely to turn to a contract development and manufacturing organization (CDMO) for process development and early-stage manufacturing. Fortunately, half of survey respondents noted that transferring the technology from an innovator’s research and development facility to commercial manufacturing at a CDMO was a smooth process. This reflects the trend toward a more collaborative approach between partners that co-develop processes.
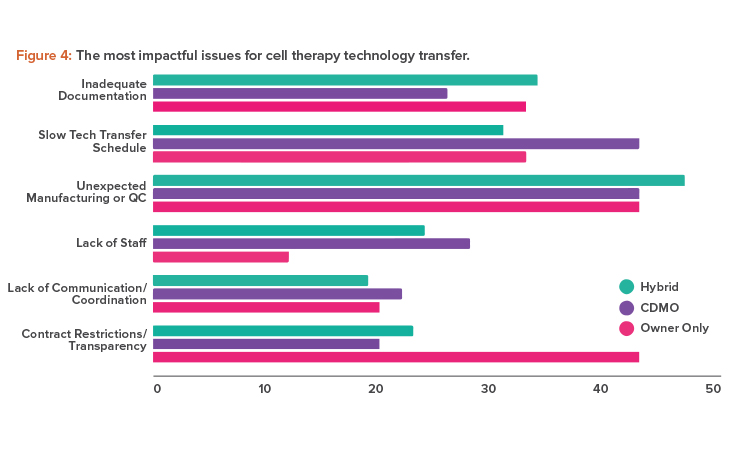
However, although only 12% of overall respondents disagreed that technology transfers were smooth; that percentage was higher among sponsor companies. They noted unexpected manufacturing changes and a lack of transparency as the most impactful issues (see Figure 4).
Unexpected manufacturing changes can occur when a CDMO continues to improve a process during development and these improvements are either not communicated to or cannot be accommodated by the client company. Problems like this can be reduced with transparency and good communication. A good example would be a sponsor company lacking the necessary technology and methodologies that a CDMO can provide.
Design in Flexibility
Designing for flexibility in the manufacturing layout can help accommodate unexpected changes and anticipate technological innovations that may evolve. Ideally, the company can rely on this roadmap to design the current process, then make future changes without investing in a complete redesign or renovations. In addition to automation and process closure, flexible design considers what equipment would need to be switched out by anticipating move-in and move-out paths, allowing changeover without having to alter the facility’s core structure.
Flexible design could include a Grade B cleanroom to accommodate an open process. Companies progressing toward a closed process may find themselves in transition and still needing aseptic processing suites for certain manufacturing steps that remain open, such as the preparation of custom tubing assemblies or small-volume sterile solutions.
Given the time-sensitive nature of cell therapy manufacturing, one way to reduce risk and increase flexibility is to bring the quality control test lab closer to manufacturing. When determining which assays will be accommodated in house vs. outsourced, companies should consider the sample handling and logistics involved, as well as the increased risks and duration of product release.
When sponsor companies and CDMOs work together, they can develop scalable, patient-focused manufacturing strategies that alleviate many technology transfer woes. Two notable trends that forward-thinking partners are embracing are developing a platform process and point-of-care manufacturing.
Adopting Platform Processing to Scale Out
Although the steps for each cell therapy process are often similar—for example, genetic material in a virus is transduced into a patient’s cells—the process, equipment, and vectors used for each treatment can be unique. The choice of vectors is diverse and includes viruses, mRNA, and gene-editing technologies. Different types of cells may be isolated from each patient, equipment may be different, or modular pieces of equipment may come together in a different order. There are also dozens of manual processing steps that take many days to complete.
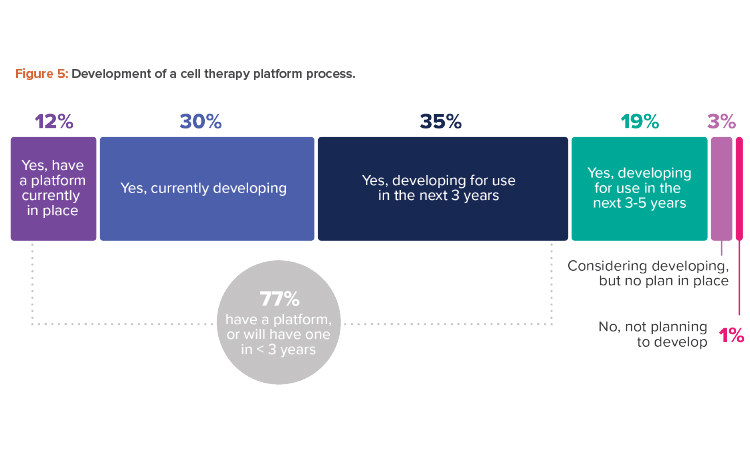
A platform process offers a reliable standard process from beginning to end across different products in the same modality, which leads to similar process steps, methodologies, equipment, and testing. Where the variation lies—to pivot from one indication to another—is at the transgene level. The industry is moving in this direction, with fully three-quarters of respondents having a platform now or planning to have one in less than three years (see Figure 5).
Companies working toward a platform process may opt to tailor their process to an available “process-in-a-box” solution or optimize it to best fit the available modular technology in the market.
Standardize
Standardization allows companies to take advantage of platform processing, which could save time and money by streamlining commissioning, validation, and regulatory approval; simplifying operator training through cross-training and interoperability; reducing labor needs and costs; and diminishing unexpected manufacturing changes during technology transfer.
A standardized, platform-based facility will give companies the flexibility to support multiple product profiles in one facility, using the same equipment and materials. It also can be customized to adapt to other variables, such as geographical location or scalability. It uses the same facility, equipment, and materials for multiple product profiles. Process-in-a-box equipment enables faster deployment and gives patients faster access to cell therapies.
Getting Ready for Point-of-Care Manufacturing
The downsides of the centralized manufacturing model revolve around complex logistics. Delicate materials often need to be moved from a patient at the clinic to an apheresis center, then to a manufacturing facility for processing, and then be transported back to the patient for treatment. In addition, there are rigorous in-process control and release testing protocols to ensure product quality and safety before it can go back to the patient. The effectiveness of a cell therapy depends on a cold chain that is vulnerable at each of these steps. Transportation introduces significant risk to the endeavor—one delay could ruin a batch.
Point-of-care manufacturing removes many of these steps, thus lowering risk and enhancing patient access while strengthening the chain of identity and chain of custody and reducing the need for a cold chain to maintain cell viability. An automated, closed-platform process enables point-of-care manufacturing and does so in less-expensive, lower-classified spaces with minimal interventions. Companies can reduce staffing numbers and training timelines, meaning operator expenses are not multiplied as the process is scaled out.
There are currently aseptic processing suites within a few major university-based medical centers, and, with time, this option could become more widely accessible. An automated process can be industrialized and potentially integrated into a process-in-a-box because it is more predictable and has better definition and less variability. Automated equipment lends itself to the promise of inline analytics and data collection to monitor quality and provide feedback to improve the process.
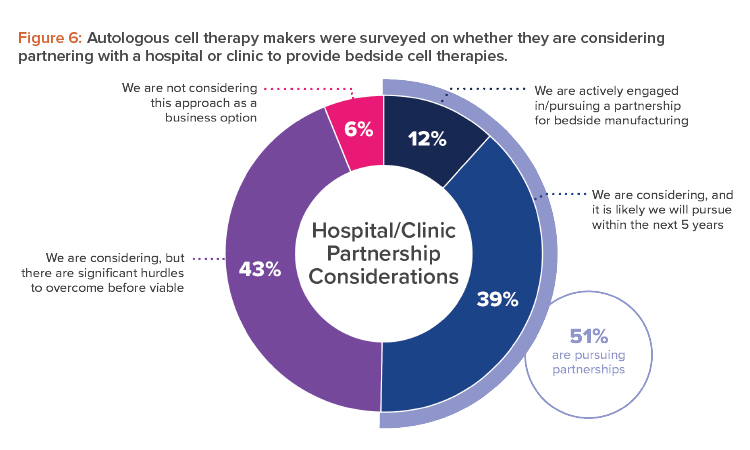
The allure of a platform process, and the point-of-care manufacturing it allows, is especially evident for makers of autologous cell therapies. Almost all those surveyed (94%) indicated they were either engaged or open to considering a partnership with a hospital or clinic to provide bedside cell therapies (see Figure 6).
If a platform process is available, the process will travel well and can be done in a smaller current GMP environment or even within or close to a medical center. This point-of-care manufacturing and delivery improves patient accessibility.
Conclusion
If our industry is going to fulfill the promise of cell therapies, significant challenges need to be addressed. Using the collective experience of hundreds of cell therapy industry experts working at innovative companies, we have identified design strategies to inform and support improved patient access to curative therapies. Designing to include process closure, using standardized process platforms, and embracing Pharma 4.0™ initiatives will have the synergistic effect of driving down costs and speeding up delivery of these life-saving therapies.
Cell therapy manufacturing is a nascent sector of the pharmaceutical industry. Although we are still a few years away from an ideal facility that embraces these technologies, cell therapy manufacturers are moving as fast as they can to apply pioneering technologies to benefit patient populations that, in many cases, are critically ill.


