Digital Display Labeling in Clinical Supplies for Clinical Trials
Digital display labels (DDLs) offer an alternative solution to eliminate manual relabeling in the clinical supply chain, optimizing label content updates through a simple, system-controlled approach while providing new, uncharted opportunities. With increased efficiency in making regulatory-compliant changes and enhanced flexibility in the clinical supply chain, DDL technology has the potential to revolutionize drug development and to increase and expedite patient access to therapies.
This article introduces a groundbreaking solution known as DDLs which have the potential to revolutionize the labeling process in the clinical supply chain for the pharmaceutical industry. Furthermore, this article advocates for industrywide alignment in adopting this solution, harmonizing its implementation, and collaborating with regulatory bodies to optimize its use in various scenarios for the ultimate benefit of patients.
The Need to Modernize the Supply Chain
As global focus on patient safety and accessible information increases, along with the need to address drug shortages and promote diversity, equity, and inclusion in the healthcare industry, there is a growing demand for the digitization of the clinical supply chain and the utilization of real-time data. The pharmaceutical industry recognizes the importance of leveraging innovative technology to streamline and expedite the development of new medicines.
However, with the rapid pace of new drug development, it is vital to modernize current clinical supply chain operations to reduce lead times and resource requirements, enhance flexibility, and minimize waste. The digitalization and streamlining of clinical supply chains will work to achieve these goals, which provides an opportunity for increased access and reduced burden for the patient.
A DDL is an electronic component with an e-paper display of the required label information, which replaces the traditional paper label on investigational medicinal products (IMPs) used in clinical trials. This digital solution enables the clinical supply chain (global clinical supplies, vendors, depots, and clinical sites) to eliminate manual relabeling. It also allows for easy and on-demand updates of label content and other information to patients and their caregivers, as well as clinical study personnel.
Although DDLs have been widely used in other industries for many years, this technology is projected to become more prevalent in the pharmaceutical industry, specifically during the execution of clinical trials. Key stakeholders such as patients, clinical study personnel, sponsors, and health authorities will benefit from the digitalization and clinical supply chain innovation, which will provide opportunities to improve patient access, safety, and the clinical trial experience.
Transforming the IMPS Label
As highlighted by a 2018 Pharmaceutical Engineering® feature, digital labels have been used in sectors outside of the pharmaceutical industry for over 40 years.1 Leveraging this technology to label investigational clinical supplies for clinical trials is a novel application. DDL in clinical supply has been in development and has undergone several prototypes, which is shown in Figure 1.
Prototype 1
In 2018, the “partial digital label” was created as prototype 1, featuring a digital expiry/retest date but with most of the clinical label content remaining static on a traditional booklet label. The digital functionality provided an opportunity to efficiently
update clinical labels with a retest date, making use of near-field communication (NFC) technology. To evaluate the technology and eliminate regulatory conflicts, a US-only pilot was conducted with prototype 1. This pilot included two expiry extension updates in different settings, one in a nonclinical setting and the other at a clinical trial site. The pilot demonstrated the value of leveraging this technology to perform a label update, significantly reducing the lead time from months to one day.1
![Figure 1: Transforming the IMPs label: A case study [2].](/sites/default/files/2024-03/0324_PE_MA_Hale_02.jpg)
Prototype 2
Lessons learned from prototype 1 enabled further innovation and opportunity, resulting in prototype 2, the “standalone digital label.” The standalone digital label included a select number of clinical label aspects: component identification number (CID), lot trace ID, quantity, and directions (administration of IMP, storage temperature, and precautions). However, this label was supported by a restricted, standalone system, which limited the software functionality and potential to scale and industrialize the solution. This solution has been tested in a nonclinical environment.
Prototype 3
Prototype 3 reached complete digital content capability by allowing full control over the digital display in text or image format through a company enterprise resource planning (ERP) system. In addition, capabilities such as paging (multipage labels) and using parent-child connected label updates (multilevel labels) were added to dramatically increase the amount and synchronization of information to be stored and displayed on the digital label. Further developments in software functionality are aimed at integration with third-party supply chain management systems to enable scalability.
Prototype 4
There is now an aspiration to evolve to prototype 4, or the “smart pack.” As a platform solution, prototype 4 could potentially enable the integration of temperature monitoring, as well as tracking and traceability of clinical supplies, while also providing opportunities to leverage digital technologies such as adherence monitoring via smart phones.
In the future, as DDL technology continues to advance, it will be beneficial to expand the content agility of the label and to broaden its physical capabilities. Furthermore, it is advantageous to address the overall environmental impact of introducing electronics into the pharmaceutical industry. This can be achieved through further developments that minimize the environmental impact of DDLs at the component level.
Additionally, to fully optimize the potential of DDLs, systems supporting clinical supply chains can be integrated with DDL update capabilities across their current reach. This means expanding the use of DDLs beyond traditional healthcare facilities and exploring new locations such as patient homes. This integration can enhance the efficiency and effectiveness of the clinical supply chain, while improving the patient experience by reducing clinical trial burden and increasing access to real-time information.
Clinical Label Regulatory Landscape
IMP labels are a regulatory requirement, but labels also play a critical role in providing clinical trial participants, caregivers, and investigators with the information needed for safe and compliant use of the investigational product. Universal guidance on labels used for IMPs in clinical trials has not been established globally.
Regulatory requirements for clinical supply labels vary from region to region. A typical IMP label may require as many as 19 different regulatory elements.3 Some requirements shared by several regions include, but are not limited to, drug name, batch or lot number, storage conditions, country-specific language, and a unique “for clinical trial use” phrase (e.g., in the US, the following CFR statement must be included: “Caution: New Drug - Limited by Federal (or United States) law to investigational use”4).
Clinical supply labels may also have additional country requirements, depending on the therapeutic modality being investigated. For radioligand therapy and cell and gene therapy trials, the label must bear radioactive symbols, cryogenic symbols, and special cautions (“contains genetically modified organism,” “autologous use only,” etc.). For personalized medicines, the label may need to include patient-specific information.
Digital Label Innovations across the Landscape
Digital innovation to streamline labeling and supply chain operations has gained interest in both the clinical and commercial space. For commercial products, information can be accessed digitally through the use of a quick response (QR) code and an external device (e.g., smartphone). This can provide health care professionals with the most up-to-date prescribing information (e.g., medication guides, package inserts) as well as with live media content, such as videos.
In the clinical space, the concept of an e-label to simplify labeling of IMPs was explored. The solution proposed for the universal paper label would include a simplified, language-agnostic printed label conveying minimal information needed for identification, safety, and dispensing. It would direct the user to the e-label/QR code for the remaining details, which would be accessible through an electronic device.
One of the challenges companies faced when engaging with health authorities to deploy the e-label concept was the reduction in content on the IMP paper label not complying with existing regulations, which require text to be visible and human readable on the label. The e-label solution relies on technology to access this information, and health authorities felt that it was not currently universally accessible and, therefore, could result in noncompliance with regulations.3
In contrast, the DDL displays all required content on the digital IMP label itself as human readable text, ensuring compliance with the regulatory re-quirements without the need for additional technology, devices, or QR codes to access the regulatory content of the label. Any QR code or additional device available would be intended to augment and enhance the user experience and supplement the compliant content currently displayed on the label, rather than replace it.
Paper Label Challenges
Alongside the limitation of paper labels being updated at clinical sites, the restricted space available on small units, like vials or syringes, leads to font sizes that are not easily readable. Additionally, the use of lengthy, multipage booklets makes it challenging to search for specific information.5 New technology and approaches provide an opportunity to improve the clinical trial experience for patients.
The utilization of paper clinical labels can pose limitations on effective management of clinical supplies. Content on paper labels is static, in contrast to the dynamic nature of clinical development. Developing, printing, and applying paper labels is labor intensive and long lead times limit flexibility when study designs evolve.
Paper clinical labels can require significant lead time to create and apply, whereas much of the study design continuously evolves, e.g., clinical time-lines, study design, drug delivery, expiry date, retest date. This requires significant agility to ensure clinical label updates are implemented at least four weeks prior to packaging and labeling operations.
Additionally, the need for relabeling to update shelf life presents a particular challenge in supply management. Relabeling must be executed in facilities qualified for manually adding labels or reworking the finished package. This process results in increased waste and poses risks to the supply chain, because units must be periodically removed to undergo physical relabeling, which can compromise the label content and is not inherently traceable.
All of these challenges are a barrier to achieving operational efficiency, due to significant relabeling work of early expiring supplies, and hinder ef-forts toward waste reduction, due to impractical label changes. Paper clinical labels limit the ability to efficiently manage supply inventory, resulting in delays and reduced patient access and presenting a barrier to achieving waste reduction.
DDLS as the Solution
Due to their dynamic nature, DDLs have the potential to add efficiencies to clinical supply chains and benefit key stakeholders, including patients, clinical study personnel, and health authorities. DDLs provide flexibility and increased agility within the clinical supply chain, improving and optimizing labeling and distribution operations. This can ultimately reduce the risk of delays and shortages and, thus, increase the assurance for patient access.
![Figure 2: Potential benefi ts of the DDL in clinical supplies [2].](/sites/default/files/2024-03/0324_PE_MA_Hale_03.jpg)
DDLs also present opportunities to enhance diversity, equity, and inclusion in clinical trials by tailoring the clinical supply label to the patient’s preferred language. With DDL, it becomes possible to easily customize the label at the site to display information in the language most comfortable for the patient.
This flexibility allows for seamless language changes based on patient preferences, ensuring clarity and understanding throughout the trial. By reducing the need for multilingual booklet labels or country-specific IMPs, the DDL also contributes to a reduction in waste, aligning with sustainability goals and promoting environmentally friendly practices. Overall, the DDL’s ability to tailor the label to patients’ preferred languages fosters inclusivity and offers an efficient and ecofriendly solution.
The fluidity of the DDL provides more real estate to increase information, which addresses patients’ needs. For example, prototype 3 (see Figure 1) has a page-flipping capability to dramatically increase the amount and synchronization of information to be stored and displayed on the digital label. This provides the ability to adjust font size, provide additional information (e.g., specific notes from the clinical site), and add universal icons, which are easily recognized by the patient, to supplement directions for use or storage conditions: for example, a sunrise to denote AM, a moon to denote PM, or a snowflake to denote the need to refrigerate. This increases patient safety and adherence, and clinical site efficiency.
Prior to implementation, ensuring the accessibility and legibility of information presented on the DDLs is paramount. Keeping the user and use en-vironment in mind throughout development, stakeholder feedback has been elicited from a variety of users, such as patients and clinical study person-nel (e.g., pharmacists, nurses) across various settings (e.g., the patient’s home, clinical and sponsor sites).
For prototype 2 (as shown in Figure 1), a legibility study was conducted on a diverse, small patient population and compared the DDLs to conventional paper label controls in both bright and dimly lit room conditions. The DDL performed similarly compared to a traditional paper label, and these outcomes serve as a foundation for further improving DDL technologies, while also highlighting the safety and effectiveness of DDLs as a viable alterna-tive to paper labels. Additional legibility studies will be conducted iteratively as design changes occur, to ensure the readability and safety of DDLs.
Input on Label Design
Patients and Caregivers
The feedback and studies aim to gather input from patients and caregivers who will be directly impacted by and interact with the DDL. They have been conducted specifically to obtain insights on the design of label content and various features of the DDL. By incorporating these inputs, the DDL can be carefully crafted with a patient-centric approach in mind, while ensuring no additional risks are introduced such as impacting health care personnel’s ability to reconstitute or administer.
The aim is to develop features that optimize the clinical trial experience for patients and alleviate any burdens they may encounter. It is anticipated that the patient insight and pharmacist and nurse studies will be completed by the end of the first quarter of 2024.
Clinical Personnel
To ensure the design of the DDL does not introduce risk or administrative burden to clinical study personnel at clinical sites, initial assessments have been conducted at three different clinical settings located in the northeast region of the US. The feedback received was positive overall, with additional considerations to ensure success in these settings. These assessments will continue to be conducted to ensure input collected represents diverse geo-graphical perspectives from different clinical site settings and patient care populations, as well as to capture differences in procedures and operations.
The use of DDLs enhances flexibility within the supply chain. Traditionally, changes in study design or protocols could have significant implications on the labeling and distribution of clinical supplies. Using the DDL, label modifications such as updating the IMPs reevaluation date (RED) (expiry date) can be made without manual relabeling, which reduces the risk of label inconsistences and errors, eliminates labor-intensive steps, and reduces administra-tive burden.
The DDL enhances supply pooling opportunities. Drug supply pooling is a method where clinical goods are centrally supplied and can be used for multiple protocols. Pooling moves the clinical supply chain from material- and labor-intensive protocol-specific packaging and labeling to a proto-col-agnostic supply chain with reduced impact to the clinical site operations.
Leveraging just-in-time (JIT) labeling, the IMP can be labeled with the assigned protocol number just before it is needed. However, JIT labeling can be further streamlined with DDLs by simply updating the digital label to reflect the assigned protocol number or any other country-specific information, eliminating the need for manual labeling.
DDLs offer the flexibility to adapt and adjust more seamlessly, accommodating evolving study requirements and timelines. This increased agility in the supply chain contributes to faster drug development and facilitates quicker patient access to potentially life-saving treatments.
A notable advantage of DDLs is their ability to facilitate documented changes efficiently and in a manner that complies with regulatory requirements. A technical prerequisite for any use case is effective system integration. The initial placement or update of the label content is monitored, executed, and controlled remotely via the ERP IT-controlled environment, which captures and documents changes and therefore does not require a qualified packaging facility.
The content on the label can be “written” on the digital display via NFC technology. Finished product can be rereleased in real time after updating via NFC technology. Executing label updates through a system-controlled approach saves on lead times, expediting supply operations from weeks to a matter of days.
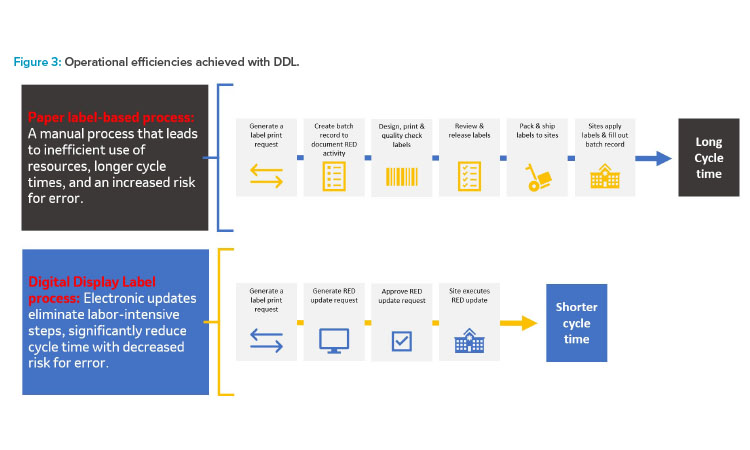
This estimated reduction is extremely beneficial when considering the supply planning for products which have a very short shelf life (e.g., radioligand therapy molecules have a shelf life of 72 hours). More DDL benefits will be presented as part of future publications. Figure 3 demonstrates how leveraging DDLs streamlines RED label updates to significantly reduce cycle times.
Looking ahead, the versatility of DDLs offers promising opportunities for further enhancing patient safety, adherence, and holistic patient care. As one example, including tracking mechanisms can enable temperature monitoring and geolocation. Integrating DDLs with other innovative technologies, such as smart packaging, allows for a shift from independent technologies to a comprehensive digital platform solution.
For instance, combining DDLs with smart dosing technologies can improve medication adherence by accurately tracking and monitoring medication adherence. Continuous patient monitoring through digital biomarkers and using digital platforms for patient engagement are also potential areas of integration with DDLs.6
By integrating DDLs with a digital platform, various patient-centric features can be incorporated. This includes personalized patient experiences by capturing and displaying specific information such as visit sched-ules, appointment and dosing reminders, patient diaries to record symptoms and adverse effects, motivational messages to encourage therapy continua-tion, timely communication of critical safety updates, and enabling direct communication between patients and clinical sites through two-way commu-nication channels. It is important to note that the development of such integrated technology is subject to additional regulatory requirements and product classification considerations.
Overall, the adaptability of DDLs opens exciting possibilities for transformational advancements that prioritize patient safety, adherence, and comprehensive patient care by integrating with other digital technologies as a platform solution.
Technology Background
To ensure reliable performance of the DDL, the development and implementation of hardware requirements should be rolled out in a phased-approach: for example, starting with a minimum viable product (MVP) that undergoes robust testing and hardware and software validation before development and implementation of commercial and reusable products. Considerations for a robust implementation strategy include addressing interoperability upfront, integrating existing standards, such as GS1.7 A target MVP should consider the following capabilities and attributes (not all-encompassing).
Package Types for DDL Integration
Consider the product portfolio and select the most prominent product formulation and primary packaging container (solutions for injection contained in a 2-mL vial, tablets that are packaged in a bottle, blister wallet, a combination product that is provided as a kit enclosed in a carton, etc.)
Image and Display Capabilities
How will the resolution and quality compare to traditional paper labels? How many and which languages need to be displayed? Consider local coding and serialization regulatory requirements such as barcoding, standards that the country follows or accepts (e.g., GS1, Health Industry Business Communications Council, International Council for Commonality in Blood Banking Automation), requirements for including ISO symbols on the product, etc.
Challenges to Adoption and Implementation
Adoption of new technology is inherently challenging, especially in a highly regulated industry. This requires socialization and collaboration across industry and with regulatory bodies to further enhance development and optimize use cases for the ultimate benefit of patients.
Investment
Like any new technology, the initial investment required for adopting and implementing DDLs is higher compared to traditional paper labels. This is due to the need for additional hardware and software, as well as the establishment of operational infrastructure. However, as the technology gains widespread adoption across the pharmaceutical industry, the increased demand and established infrastructure will eventually lead to cost reductions.
Failures
Although there is the potential for breakage of the DDL, paper labels are also prone to failures such as the ink fading, flaking off, or the label itself falling off. The key is understanding such risks through testing in various conditions, addressing risks, and validating the integrity of the label to provide a higher level of confidence in its use.
Waste
Although aiming for a sustainable solution, implementation of a global approach for the collection, reprocessing, and reissuance of DDLs to enable reuse of the technology can be challenging.
Software and System Integration Requirements
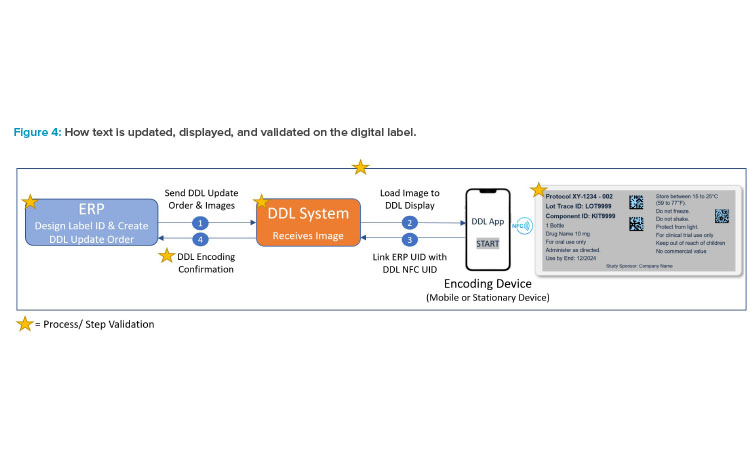
The steps to creating a label remain the same, whether the label is displayed on the DDL or printed on paper. For DDL, there is communication between the ERP system, where the DDL image is generated, and the DDL system, where the image is loaded and then displayed on the digital label (shown in Figure 4). Validation ensures that the DDL is secure, and final release of the approved digital label text is performed per normal label release procedures.
Any digital label solution will require mapping of unique identifiers (of the digital label and the data source). It will also require verifying that the DDL system is GxP validated and Part 11 compliant; the label always displays correct image for user acceptance testing (UAT); and that during the label image update, positive encoding confirms DDL was updated correctly. Figure 5 gives an illustration for such identifiers using a mobile phone as a meta-phor in this example.
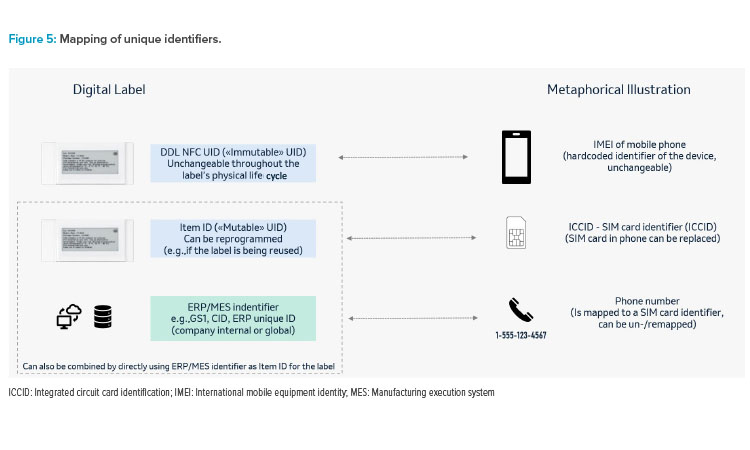
In more centralized system landscapes, this could consist of only two levels by writing the ERP/MES identifier as “mutable” unique identification number (UID) to the label and directly mapping the source to the label. In more complex environments (e.g., if there were parallel data source systems), there may be a need to have a third level in between, hence mapping the ERP/MES ID to this item ID in the source system(s), and the item ID to the NFC UID in the digital label system.
It is probably sensible to distinguish use cases between the two perspectives of the source system and the digital label system. The standard use cas-es to be implemented in the digital label system are relatively few (initial load, update, scrapping individual units, recycle the label), potentially with some additional functions to handle exceptions. From a source system(s) perspective, the use cases (triggers) may be a lot more versatile, but usually result in one of the standard use cases at the digital label system end. While in development, the goal is to have the DDL system available as a commer-cial off-the-shelf software, compatible across computer systems.
Conclusion
Although DDLs offer a wide range of benefits to patients, clinical trial personnel, sponsors, and health authorities, certain challenges must be addressed to realize the full benefits. These challenges include obtaining industry and regulatory acceptability, upfront investment costs, and implementing a global approach for reuse.
With increased efficiency in making regulatory-compliant changes and enhanced flexibility in the clinical supply chain, DDL technology has the po-tential to revolutionize drug development and to increase and expedite patient access to therapies. The transformative capabilities of DDLs, with op-portunities for integration with other digital technologies to deliver a platform solution, pave the way for more efficient and patient-centered clinical trials in the future.
Acknowledgements
The Clinical Supply Leadership Forum (CSLF) is a pharmaceutical industry global advisory group that is committed to providing leadership and strategic direction through the establishment of an open discussion on precompetitive critical technical issues and challenges; identifying opportunities for alignment, innovation, and promoting consistency; and seeking worldwide harmonization, where applicable.
The Digital Display Label Community of Practice (DDL CoP), as part of the CSLF, is dedicated to harmonizing the industry application of DDL for clinical supply, creating benefits for patients, health authorities, clinical sites, and sponsors.
DDL CoP Authoring Team
• Jessica L. Hale, PharmD, MSD
• Raj Shah, PharmD, MSD
• Alexander Debets, PhD, MSD, Switzerland
• Martina Marauli, MSD, Switzerland
• Kristel Rens, Janssen Pharmaceutica NV, a Johnson & Johnson company
• Pieter Piron, Janssen Pharmaceutica NV, a Johnson & Johnson company
• Rachel Owen, Astra Zeneca
• Matt Bolton, MSD
• Julia Hagan, Eli Lilly and Company
DDL CoP Reviewers and Other Contributors
• Urs Baumann, MSD, Werthenstein BioPharma GmbH
• Giuseppe Coppola, Novartis Pharma AG
• Vismita Kandasamy, PharmD, MSD
• Nicohle J. McGeorge, Bristol Myers Squibb
• Nancy Morrone, Pfi zer
• Rob Pizzie, PhD, Brizzey LLC
• Frank Reale, RPh, MSD
• Ashish Shedage, Novartis
• Divyendra Singh, MSD, Czech Republic
• Jessica Sonevytsky, MPH, MSD
• Christina Thommen, Novartis Pharma AG
DDL CoP Members
Alexion, Astra Zeneca, Bristol Myers Squibb, Brizzey LLC, Eli Lilly and Company,
Johnson&Johnson, MSD, Novartis, and Pfizer


