The ISPE Pharma 4.0™ Operating Model’s - Pharma-Specific Maturity Index
ISPE’s initiative to transform industry-generic 4.0 models for pharma operations will help the industry benefit from digitalization.
In today’s pharmaceutical industry, the landscape of operating models is heterogeneous, and the consequences of this heterogeneity can hinder progress. For example, the risk-based regulatory approach, in which regulators and companies proactively collaborate to prevent harm, is ready for use, but the industry lacks the tools and methodologies to demonstrate that there is a strong business case to adopt the approach.
Instead, the structural capabilities of pharmaceutical companies are mostly driven through regulatory requirements, and pharmaceutical regulation is narrowly focused on the current processes for marketing authorization approval, manufacturing, and postmarketing obligations through good practice (GxP) compliance activities.
Fortunately, digitalization opens new horizons for a holistic perspective on business. It can provide accurate information for decision-making that will allow the pharmaceutical industry to achieve new levels of connectivity, transparency, agility, and productivity. At present, digitalization in the pharmaceutical industry is immature. Adoption of global standards and concepts such as those coming from the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) is in progress. However, in some cases, the demands of the digitalized value network, stakeholders, and patients are still not being met. Specifically, current knowledge and tools to monitor the ICH control strategy throughout the global product life cycle do not completely allow for continuous improvement activities. In addition, the current environment is not allowing regulators to have a more digitalized overview of the pharma industry.
ISPE’s Pharma 4.0™ initiative aims to help the pharmaceutical industry overcome the obstacles to digitalization. Pharma 4.0™ draws from an industry-generic Industry 4.0 Maturity Index from the German Academy of Science and Engineering (Acatech) to shape the environment of future pharmaceutical operations. The pharma-specific Pharma 4.0™ Operating Model describes exemplary key enablers and elements of essential importance in the pharmaceutical operations processes, environment, and culture.
The various stakeholders in Pharma 4.0™ have distinctive perspectives and motivations:
- Strategic point of view (e.g., from CEOs or business unit heads)
- Technical operations and quality risk management (QRM) point of view (e.g., from heads or experts of technical operations, production or engineering)
- Information technology (IT) point of view (e.g., from IT or engineering heads or experts)
Whichever perspective they hold, all stakeholders share a common expectation that digitalization will help their companies achieve business goals by operating faster, reducing costs, and being more competitive and agile.
This article will focus on the technical operations/QRM and IT perspectives without neglecting the other perspectives. The Pharma 4.0™ world is absolutely determined by a holistic view of the full value network and building digitalized end-to-end business processes. Connectivity and impact have never mattered more than now in the world of Pharma 4.0™. Boundaries will become increasingly irrelevant in all dimensions.
Vision and Mission of Pharma 4.0™
The following are the principle tenets of the Pharma 4.0™ vision:
- Digitalization will open new horizons to achieve new levels of connectivity, transparency, agility, and productivity through the application of faster and more accurate information for decision-making.
- Functional silos will no longer exist after digitalization is successfully implemented.
- Globally harmonized GxP rules and technical standards will be in place. Mutual recognition of inspections will occur more frequently. In addition, enforcement of rules and standards will be driven equally by current industry practices and by digitalized and connected computerized systems.
- Regulatory authorities are essential partners in reaching this goal. Industry–regulatory agency partnerships are critical to design a win-win situation for all stakeholders in the pharmaceutical value network.
- In a Pharma 4.0™ world, it will be feasible for stakeholders to be connected, while respecting and applying different cultures and management styles.
The ISPE Pharma 4.0™ special interest group (SIG) created the following mission statement to pave the way for the regulatory Pharmaceutical Quality Initiative of the digitalized 21st century:
“Manufacture pharmaceutical products with maximum product and process understanding, data integrity by design, efficiency and optimal resource allocation on the basis of full digital data transparency—to the benefit of the patient.”
Developing a Pharma-Specific Maturity Model
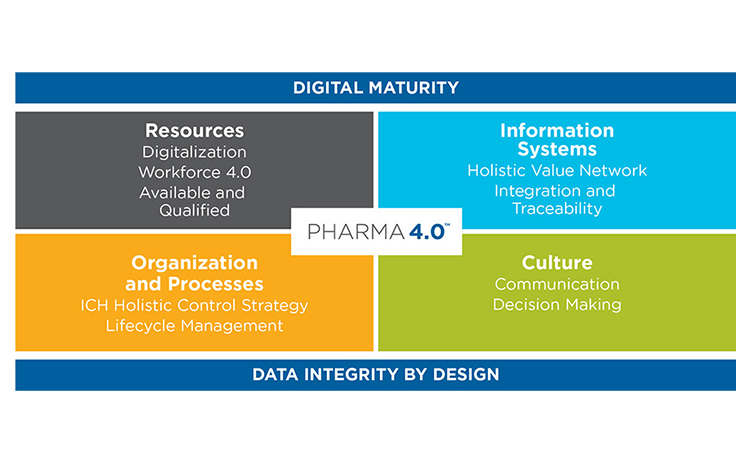
The mission of the Maturity Index subgroup of the ISPE Pharma 4.0™ SIG is to develop a pharma-specific maturity model, based on the four elements of the ISPE Pharma 4.0™ Operating Model (Resources, Information Systems, Organization and Processes, and Culture) and two enablers, Digital Maturity and Data Integrity by Design (see Figure 1). The ISPE Pharma 4.0™ Operating Model, including its enablers and elements, has been described in greater detail in past issues of Pharmaceutical Engineering.1 , 2
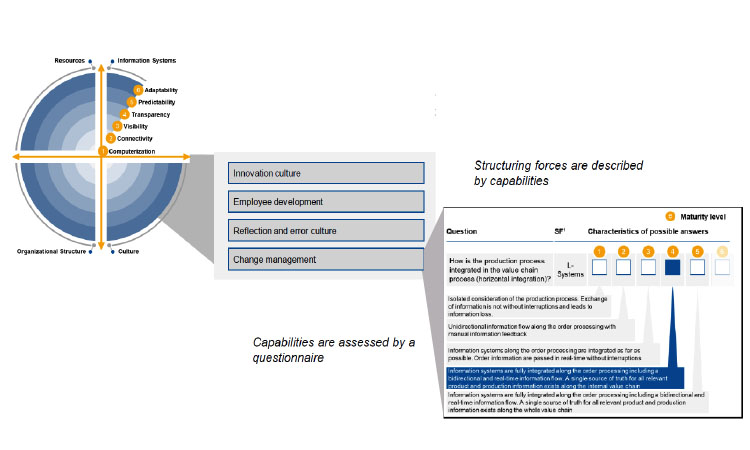
The ISPE Pharma 4.0™ SIG offers a cross-functional working platform for relevant experts representing stakeholders to work on a volunteer basis in a formalized environment and at low-resource consumption to create new content and new best practices. When first developing Pharma 4.0™, the SIG looked for resources on operations intelligence developed elsewhere, and used the methodology for an industry-generic maturity model developed by the Industry 4.0 Maturity Center at RWTH Aachen University in Germany as a starting point (see Figure 2).
An important asset of the industry-generic model is its Maturity Index, which clearly identifies objectives and building blocks for six maturity levels on the path from digitalization to industry transformation:
Level 1: Computerization—At this initial level of digitalization, the objective is to simplify repetitive tasks. Building blocks include introducing IT on the shop floor and elsewhere.
Level 2: Connectivity—At level 2, the objective is to streamline business and IT. Building blocks are the digital connection and integration of business practices.
Level 3: Visibility—The objective for level 3 is to make databased decisions. The primary building block is the construction of a real-time digital shadow.
Level 4: Transparency—At this level, the objective is to grasp complex interactions through the building blocks of running data analytics and interpret the findings to understand effects.
Level 5: Predictability—At level 5, the objective is to prepare for upcoming situations. The key building block is the capacity to simulate possible future scenarios.
Level 6: Adaptability—Finally, the objective for level 6 is leave the control to the system. Wherever possible, the building blocks for this level should allow the system to adapt itself to new scenarios.
| ICH Q10 PQS | Pharma 4.0™ | |
|---|---|---|
| Elements | Corrective action and preventive action (CAPA) system Change management Management review | Resources: Digitalization, available and qualified workforce 4.0Information systems: Holistic value network integration and traceability Organization and processes: Holistic control strategy, life-cycle management Culture: Communication and decision-making |
| Enablers | Knowledge management Quality risk management | Digital maturity Digital integrity by design |
Pharma specificity was added to this generic maturity index by applying the principles of ICH Q10: Pharmaceutical Quality System (PQS), a model that defines the key enablers and elements as required from a pharmaceutical and regulatory perspective throughout the product life cycle and across the value network. Furthermore, additional elements and enablers were defined based on the Pharma 4.0™ Operating Model (see Table 1). Figure 3 shows how the objectives for levels 1–6 of the industry-generic maturity model fit with the Pharma 4.0™ Operating Model.
The method to characterize elements of Pharma 4.0™ can be applied similarly to a description of a business process, a production process, or a computerized system or network. The term “enablers” in the ISPE Pharma 4.0™ model is allotted to “Digital Maturity” and “Data Integrity by Design” because these enablers are considered most relevant for pharmaceutical operations and throughout the pharmaceutical life cycle. Readers should note that the usage of the term “data integrity” in pharma operations varies from its general meaning in IT. In pharma operations, it is a regulated term enforced in GxP inspections by regulatory authorities.3 The addition of the phrase “by design” suggests that data integrity is embedded throughout the internal and external systems architecture.
Maturity Levels for the Four ISPE Pharma 4.0™ Operating Model Elements
The following sections review examples of how the Pharma 4.0™ maturity index may apply within each of the four elements of the Operating Model: Resources, Information Systems, Organization and Processes, and Culture. The highlighted capabilities are key capabilities, but they are not an all-inclusive list.
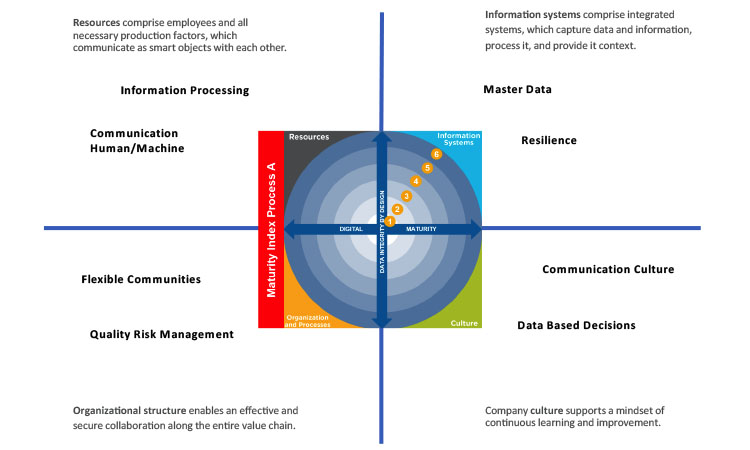
Resources
Resources are tangible, physical assets. They include a company’s workforce (human resources), machinery and equipment, tools, materials, and the final product.
Information Processing
In information processing, the maturation of resources may begin with a manual pull from different computerized systems, or even paper-based sources, to create databases that interact via sensors and actors (e.g., the “one data warehouse” concept). As information processing resources mature further, they can provide a digital “shadow” of processes to achieve high visibility in the connected shop floor.
The next levels of information processing will connect all relevant functions in cross-functional business processes from along the complete supply chain, from drug substance/active pharmaceutical ingredient (API) development via drug product formulation and packaging and commercial manufacturing to the patient. A holistic view of these processes will allow for more transparency.
More mature levels of information processing are achieved when QRM is actively supported by digitized systems. For example, a corrective action and preventive action (CAPA) system available throughout a corporation could enable experts to obtain a global view of all events connected with their own process that occur at other places. The most mature level is achieved when a system is leading the process of its own adaptation (e.g., the corporate CAPA management system is linked to the decision-making process).
Communication Between Operator and Machine
Initially, communication between operator and machine requires a worker’s physical presence at the machine. As communication matures, workers can make adjustments via a remote interaction mode. The next step is to make all digitalized information from the machine available to all employees concerned. Digitalized and standardized documents should be available for all relevant users. Also, critical information, such as reports, and warnings should be generated by the system and available 24/7 so that the responsible persons can intervene at the earliest possible moment. At the next level of maturity, all relevant functions around the machine (e.g., environmental control or preventive maintenance) are integrated. A fully mature system can lead quality-driven decisions in the manufacturing shop.
- 1Binggeli, Lorenz, Hans Heesakkers, Christian Wölbeling, and Thomas Zimmer. “Pharma 4.0: Hype or Reality?” Pharmaceutical Engineering 38, number 4 (July–August 2018): 40–4.
- 2Herwig, Christoph, Christian Wölbeling, and Thomas Zimmer. “A Holistic Approach to Production Control—From Industry 4.0 to Pharma 4.0.” Pharmaceutical Engineering 37, number 3 (May–June 2017): 44–9.
- 3ISPE GAMP Guide: Records and Data Integrity. Tampa, FL: ISPE, 2017. See also US FDA. Data Integrity and Compliance with Drug cGMP: Questions and Answers Guidance for Industry. https://www.fda.gov/downloads/drugs/guidances/ucm495891.pdf; MHRA. ‘GXP’ Data Integrity Guidance and Definitions. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/fi le/687246/MHRA_GxP_data_integrity_guide_March_edited_Final.pdf
Information Systems
Information systems are sociotechnical systems in which information is provided based on economic criteria determined by people as well as by information and communication technology. Information systems prepare, process, store, and transfer data and information.
Master Data Management
Starting from a situation where master data are created and maintained “in an island,” the initial step toward maturation in master data management is likely driven by new regulatory requirements for data integrity (e.g., US FDA, World Health Organization, or Medicines and Healthcare Products Regulatory Agency requirements). Under these requirements, companies must create a companywide or value-chain-wide master data framework, and certain types of data (e.g., information about excipients and APIs) must also be globally and publicly available. Each substance must have a specific name and a quality-specific reference number. Master data management can then mature to capture and manage all pharmaceutical product-specific data, allowing for system supported life-cycle management from product development to the transfer to production to the phase-out from all markets at the end of the life cycle. At the most mature level, the system could lead product life-cycle decisions in manufacturing, quality, supply chain, and business.
The process of maturation involves transitioning from a hierarchical organization, with separated functions, to an agile organization without silo-supporting structures.
Resilience of Information Systems
Companies may start from a situation where IT systems are manually configured and key systems do not have redundancies. These systems comply with US FDA Part 11 or EU Annex 11 requirements for validation of computerized systems in the sense that they work without any major deviations.
A maturing information system can isolate failures to avoid a cascade of failures to other systems. This is known as computerized system validation and compliance under control between systems, or data- ow control between systems.
The subsequent level of maturity is achieved when performance statistics are automatically generated and the integration of new servers can be easily reproduced and completed. The higher levels are described as the availability of a catalog of failures that supports a quick identification of failures and remediation. Finally, a mature system automatically generates operational reliability risk analyses and reports, thereby providing access to the digital world to inspectors.
Organization and Processes
“Organizational structure” refers to both a company’s internal organization (structure and operational processes) and its position within the value network. The organizational structure establishes mandatory rules that organize collaboration both within the company and externally.
Flexible Communities
The process of maturation involves transitioning from a hierarchical organization, with separated functions, to an agile organization without silo-supporting structures. In a traditional organization, there are communication hurdles between the silos and between the hierarchical levels. The worker receives goals from the line manager, who also evaluates the employee’s performance.
In theory, a fully agile organization can be established with self-organized teams, which have end-to-end accountability for their product. Squads, tribes, chapters, and guilds are the organizational elements. There is neither a classical hierarchical structure nor a single chain of command—just business goals. All organizational elements may be formed and disbanded spontaneously, or they may be established for the long term. Each employee can work in several squads and contribute to chapters and guilds. They are temporarily assigned to these units. Employees are responsible for finding assignments that interest them, and an assignment can last from several months to several years. Employees have a set of goals for each of their assignments. Their performance is evaluated by the units they contribute to or by an owner of the total optimum/business goal.
Quality Risk Management
When considering the maturity of QRM, the key question is to what extent does the prevailing risk culture support compliance with the defined quality standards? On the immature end of the spectrum, there is limited or no risk communication among the different functions and organizations, and no training for risk management. In contrast, in a mature system, risk governance and information security enable trust in risk communication in the value network. Risk events are regularly detected and communicated in the value network to allow organizations to react in time to even weak signals of distress.
Culture
Culture is the value system within a company and thus describes the “soft” aspects of collaboration. The structural areas of organization and culture are mutually dependent and must be in agreement with each other.
Communication Culture
Starting from a situation where records are versioned, documented, approved, and simply accepted as facts, there is a long way to go to reach more mature levels of communication related to data. Generally, the path to transformation is the way from a “need to know” and “hierarchically controlled” culture to an “open” and “collaborative” mode of work.
Ultimately, an improved communication culture has a knowledge management system in place and an owner for the total optimum of the individual optima from individual process owners. Additionally, a dispute resolution process is installed and regulatory responsibilities are fully integrated in the decision-making system or process. Digitalization can play a key role in achieving such high levels of communication.
Data-Based Decisions
In contrast to situations where decisions are made based on the knowledge or intuition of individuals, a more mature communication culture allows expert teams to make decisions based on data, with historical data supporting their choices. A deep data analysis represents the next level of transformation. The highest-level decision-making processes are supported by automated data analysis and provide the possibility to run simulations and generate scenarios (Decision Making Automation). Digitalization can also play a key role in this aspect of communication culture.
Outlook
The ISPE Pharma 4.0 SIG and its Maturity Index Working Group are continuing their work to create a Maturity Index to help pharmaceutical companies benchmark the level of digitization they have achieved and identify focus and development areas to fully transform into a digitalized organization. In its industry generic model, Acatech considers structural areas and business processes and systems. For pharmaceutical operations, in general, the processes identified in that model exist; however, the pharma industry has certain distinctive priorities, including those shaped by regulatory requirements such as GxP inspection management and drug submission–approval processes. Additionally, driven by ICH, there are processes for life-cycle management of pharmaceutical products that are truly cross functional, as they include all functions and stakeholders from the value chain (i.e., the value network). These functions include development, product transfer units, production, quality control, quality assurance, engineering, supply chain management, marketing, and sales. In addition to pharmaceutical companies, they may involve third-party labs and manufacturers and all service providers.
Author: Sebastian Schmitz and Christian Wölbeling not in iMis


