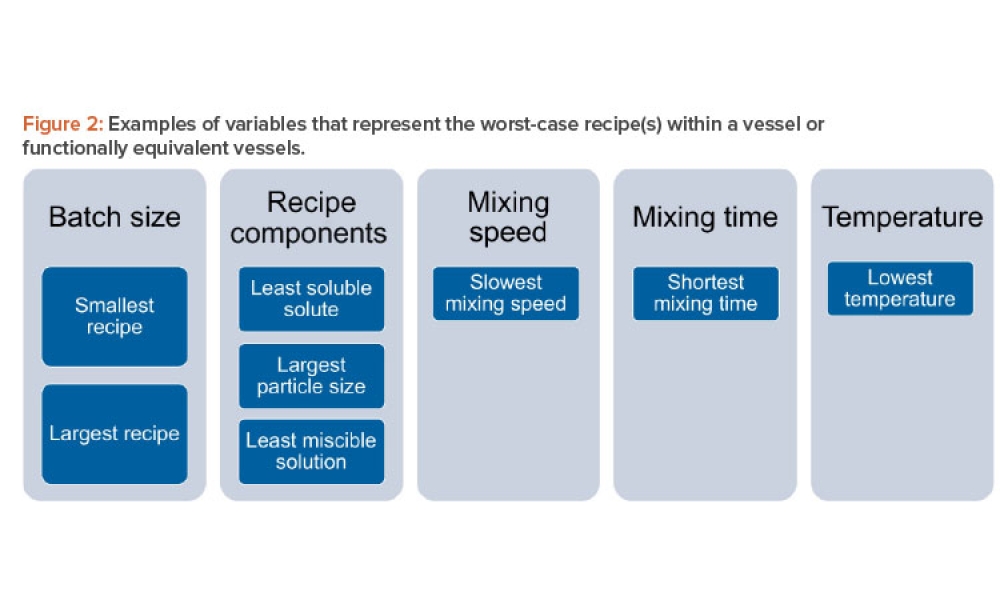
Practical Regulatory & Industry Issues

Presenters and a panel of FDA regulators and industry experts discussed key regulatory and industry issues during the closing plenary of the 2019 ISPE Biopharmaceutical Manufacturing Conference.
Advanced Therapy Medicinal Products
Peter Marks, MD, PhD, Director, Center for Biologics Evaluation and Research (CBER), FDA, spoke on “The Critical Role of Manufacturing for Advanced Therapy Medicinal Products” (ATMPs). These products include gene therapies and xenotransplantation products, as well as human cells, tissues, and cellular and tissue-based products (HCT/Ps) requiring licensure. A controlled manufacturing process and an understanding of critical quality attributes for these products provide clinical benefit, he noted.
The Advanced Therapy Medicinal Product market is growing, as indicated by the rise in investigational new drug (IND) applications to the FDA, Marks said. These new drugs present regulatory challenges because the scientific basis underlying the drugs’ efficacy is not always clear, it’s challenging to ensure adequate control of the manufacturing process without being excessive, and there’s a lack of extensive regulatory precedent in some areas (such as 3D cell printing).
Advanced Therapy Medicinal Products present clinical market development challenges as well, he said. These challenges include products intended for use in very small populations, target populations dispersed geographically from the manufacturing site of personalized therapies, and the potential need for long-term safety and efficacy data.
Manufacturing challenges for Advanced Therapy Medicinal Products include that these products are often made from living organisms and may not be easily characterized; also, they are frequently temperature sensitive and susceptible to microbial contamination, and their complexity for manufacturing facilities and processes is relatively high.
The FDA is helping advance the development of cell and gene therapy by providing guidance documents and reducing administrative burdens. The FDA also supports clinical development initiatives, standards, and manufacturing initiatives. Draft guidance on cell and gene therapy from July 2018 are available.
Expedited programs for FDA consideration are also helpful. Marks described the regenerative medicine advanced therapy (RMAT) designation to expedite product development and review. It applies to certain cell therapies, therapeutic tissue engineering products, human cell and tissue products, and combination products, including genetically modified cell therapies and gene therapies producing durable effects. To obtain RMAT status, products must be intended for serious or life-threatening diseases or conditions, and preliminary clinical evidence must indicate the product’s potential to address unmet medical needs. The FDA replies to RMAT designation requests within 60 days, and designated products are eligible for priority review and accelerated approval as appropriate.
Sponsors can fulfill postapproval requirements by submitting clinical evidence and studies, patient registries, or other sources of real-world evidence such as electronic health records, an agreed-upon collection of larger confirmatory datasets, or postapproval monitoring of all patients treated with said therapy prior to therapy approval.
As of 1 May 2019, 34 products out of 100 requests have been granted RMAT status, Marks said. Most are cellular therapy or cell-based gene therapy products.
The Initial Targeted Engagement for Regulatory Advice on CBER Products (INTERACT) program further encourages early interaction between regulators and sponsors and replaces the pre-pre-investigational new drug meeting process regarding preclinical, manufacturing, and clinical development plans.
Closed manufacturing systems are a possible solution on the horizon, Marks said. They could facilitate more efficient technology transfer, which in turn could streamline preclinical evaluation required for first-in-human trials, make technology more accessible to academic innovators, and increase the value of the asset to investigators and industry. Potential challenges include agreement on vectors, prework needed to develop vectors and protocols, and vector and protocol distribution.
Changes to Biologics License Applications
Keith Webber, PhD, Vice President, Biotechnology, Lachman Consultant Services, Inc., spoke on “Navigating Your Way from Route 361 to Route 351.” The title refers to the transition from Public Health Service Act (PHSA) Section 361, which was established to prevent the introduction, transmission, or spread of communicable diseases from foreign countries into the US or among its states, to PHSA Section 351, which specifically addresses biologic drug regulation.
The introduction of new modalities raised questions about whether they are regulated under Section 361 only, or if additional licensing is required. HCT/Ps that don’t meet all Section 361 criteria are not regulated solely under that law. A valid biologics license is needed to market a drug that is also a biological product.
Guidance issued by the FDA on 17 November 2017 provides regulatory discretion for three years, until 17 November 2020. After that date, an investigational new drug or an approved biologics license application (BLA) will be required to distribute products designated as drugs.
Section 361 compliance touches on a host of activities, which Webber outlined, including site registration, listing products, evaluating facility design, potency assays, and establishing test methods and acceptance criteria for incoming components, in-process controls, and lot release. CBER’s INTERACT program can provide advice through the process.
Panel Discussion
The Industry and Regulatory Panel discussion closed out the conference. Participants were:

A number of products are coming. What plans does the FDA have to handle the dramatic increase in the number of applications?
Marks: “We are staffing up. It’s a little bit of challenge since everyone is looking to staff up at the same time. To have somebody truly able to give feedback, they need to be at the agency for several years, and trained. Even independently reviewing takes a year or two. As new meeting types come like INTERACT Tech Team meeting (which is also new and similar to CDER critical path meetings), they are useful; but if we don’t have enough staff, it takes a long time to schedule. We will do our best and hope the industry will be sympathetic.”
One slide [RMAT applications] showed a relatively significant number of applicants were declined: 56%? Comment to general reasons.
The declined applications fall into two major categories, Marks replied. “We sometimes get people who are very excited and want the designation based on very little evidence. If you have very little evidence but it is consistent and clear, we’ve given the designation based on a handful of patients.” However, without consistency, the designation will not be given. If sponsors come back with more data, their applications may be approved.
The second, less-common reason that applications are denied is that “people submit clinical data about a product they intend to make in the future, like a product made in Europe. If you are not making the product, we don’t know you will get same results as they got in Europe. Data must be from the product you intend to use. Importing the European product is not a problem. It is not too different from breakthrough therapy designations.”
We often see clients who immediately want to get to market space but really struggle because of legacy platforms and analytics.
Application of data analytics on AI [artificial intelligence]: What is the FDA stance, especially on control strategies, testing, tech transfer, scale-up, and validation? Are guidelines coming from the FDA on AI?
AI “is real and it’s here,” Baker said. “It is not science fiction. One challenge is that with a sort of bleeding edge technology, frequently there is proprietary material, so it is difficult to have a lot of shared learning.” However, Baker shared good news: Global regulatory agencies are becoming more comfortable using model systems. “Very effective models make really good predictions.” For example, nested models can make predictions in an information space. “Conceptually, we’re comfy with that. The challenge [is to] demonstrate that the model does in fact make informed decisions.”
Another challenge with AI involves quality management (QM) systems, which are often set up as pass/fail, Baker added; however, this is not an FDA issue. “What are you going to use the system for?” he asked. “Process control? Reproducible and anticipated outcomes? If you use it for continuous improvement and platform development, how do you sequester through the quality management system? It’s a tool to make decisions. What kinds of decisions are we making? High-quality decisions come from high-quality information. It’s emerging tech.”
Marks added that the FDA met recently with Friends of Cancer Research to address chimeric antigen receptor T cell immune therapies, discussing critical quality attributes and hypothesizing outcomes if AI were used to identify those attributes. They are working to establish an agreement that encourages manufacturers to provide data, but information about critical quality attributes tends to be closely guarded in manufacturing. He noted two perspectives within the FDA. Some believe, “AI may be such a big mess that no one will figure it out.” Some take the opposite view. “Others say AI is pretty powerful, so something can be figured out.”
How to make the leap from a very small patient population, developing clinical materials, to commercial manufacturing?
McShane, who works with small firms on this challenge, replied: “When they get breakthrough designation or RMAT, the pace picks up incredibly. A lot of firms may not even have a head of quality at that point! Yet they know they need a quality system that will be commercial in less than 18 months. For a lot of my clients, quality is behind and it’s really tough to catch up. You do not want to be the company that can’t come to market because you have data integrity problems, or are not following ICH guidelines, or can’t get investigations or change control done. I suggest that everyone have a developmental plan. I urge you to have a quality plan to think about your quality system and when is the right time to put in certain segments. You’ll be way ahead going forward.”
Snyder added, “That’s where working with a CDMO [contract development and manufacturing organization] with a track record can be a benefit. A challenge we often see is clients who have gone in and licensed a technology out of a university, have proof of concept, and then immediately want to get to market space but really struggle because of legacy platforms and analytics; the cost to switch is extreme.”
Webber agreed. “Moving from earlier R&D culture to earlier manufacturing of drugs culture: it is a huge challenge to have a quality management system. It is a long-term challenge, scaling out to manage a number of patients in the future.” Development of standards was talked about at this conference, he noted, and that area will contribute substantially to development over the long term.
Magnitude of conversion to biologics license application: How many products are eligible/need to be converted? What progress has been made?
Baker did not have numbers to share. However, he noted, “The number is not so large as the scope of the challenge. Transition products have been on the market for a long time by companies committed to continuous improvement and stabilization with modern analytics—an administration exercise is what it will be.”
He also said, “The scope is broader than you might think—products and technical stewardship, and the commitment to technical stewardship shown over the last 20 to 25 years. We’re not in the business of whopping people over the head with a biologics license application stick.” On the other hand, sponsors should “stay current, appreciate that 21st-century technologies are an expectation.” He continued, “The team is working very hard on this [conversion to biologics license application]. I have been a little surprised. I expected a lot of industry engagement; I have not seen as much of that as I thought. Maybe this is because the requirement was part of the Affordable Care Act [ACA], so maybe people were waiting to see what would happen.”
In response to a follow-up question asking for an explanation of the transition process, Baker said, “When biotech was smaller, many products we call ‘biotech’ today were approved as NDAs [new drug applications], and many were approved as biologics license applications. The field was being invented.”
Then, an Affordable Care Act provision amended the definition of a biologic, stating that proteins are biologics. The FDA subsequently provided statutory interpretation of the definition of a protein. Under this interpretation, “protein products approved as new drug applications will be deemed biologics license applications,” Baker noted. These are the transition products. “Insulins are a classic example, but many others are affected.” Today, he explained, it’s easier to tell what is under CDER’s purview and what is overseen by CBER. There are differences in the two approaches: one involves approval of a molecule; the other involves licensing a manufacturing capability.
Doleski said CDER regulates some biologics; many others are under CBER’s authority. As the transition concludes, organizational responsibility within the FDA for these products will presumably change.
Baker explained, “We’re migrating all those transition products from the small molecule side of CDER for review—supplements, inspections, continuous improvement opportunities. Those have already been moved into the Office of Biotechnology Products. We’ve identified all reviewers. Many companies were given opportunities for informal meetings with new reviewers and to visit some sites; there have been many positive, informative meetings and discussions outside the context of specific decisions. There have been very different types of discussions around things like biopotency and facility issues. It’s been positive. I do not anticipate an enormous flash.”
Can you discuss going into process performance qualification (PPQ) batches with only one engineering run?
Baker said, “A biologics license application submission and process validation program are exercises in advocacy, not forensics. Any groups that approach them as forensics are well intentioned but not working within the current paradigm. You’re advocating for a large molecule biotech product and claiming you are capable of maintaining a reliable, consistent drug supply for the patient. Engineering runs mean different things at different companies and may have occurred outside quality management or off protocol. They can be supportive evidence, assuming a quality management system is live and does what it is supposed to do.” He explained that the number of engineering runs, process verification (PV) runs, and the scope of continuing validation are all part of making that argument. Professional scientists and engineers are responsible for making the case that the site is going to be ready to run. “Great development, tech transfer, process performance qualification, maintenance of validated state—all make the case,” he said.
McShane added that, per FDA 2011 Validation Guidance, PV begins with development, which can include engineering runs and process performance qualification batch completion, and continues through a continuous process verification (CPV) program.
Baker noted, “Then you are in protocol, not experiment. Protocol says, ‘This is what success looks like.’ If you’re going to run experiments to see how process works, that’s development. If you have validated process, you are providing high-level assurance that it will meet predetermined expectations in a way that impacts the patient.”
McShane continued, “FDA guidance says use statistics. After PPQ batches, start continued process verification and run it forever. Many have struggled getting continuous process verification up and running.” Ultimately, he explained, reliability over the manufacturing life of that product is what counts, and continuous process verification supports that.
Disclaimer
This is an abridged, unofficial summary of FDA regulators’ presentations and discussion during a panel dialogue. It has not been vetted by the agency. This article offers an informal and brief synopsis of the FDA regulators’ views and does not represent official guidance or policy of the FDA.
Acknowledgments
Thanks to Andre Walker for assistance with articles about the 2019 ISPE Biopharmaceutical Manufacturing Conference.