Applying Holistic Control Strategy in Pharma 4.0™

Applying emerging technologies can lead to more robust and flexible manufacturing processes that in turn can help the pharmaceutical industry respond to drug shortages, reduce interruptions in production and delivery of medicines, ensure consistent clinical performance of products, and achieve other benefits. Although some may believe that regulators are averse to the use of emerging technologies in the pharma industry, the previous sentence summarizes the position expressed by representatives of the US FDA during the 2019 ISPE Europe Annual Conference in Dublin this past April. The ISPE Pharma 4.0TM Special Interest Group (SIG) has designed an operating model as a framework for how digitalization can be applied to ICH Q8–Q12 and named it the “holistic control strategy.” This article introduces the strategy and presents two case studies of companies that have started to apply it.
The 20th century was the era of blockbuster pharmaceuticals. Given the nature of homogeneous production in large quantities for a long period of time, the pharmaceutical industry unsurprisingly adopted the principles of mass production. For more than a century, organization, culture, information systems, and resources were optimized to fulfill the rules of Pharma 2.0, which is dominated by a culture of hierarchy and experience-based decision-making and organized by functional silos. In Pharma 2.0, resources are focused on the performance of repetitive tasks, and information systems require many human interventions, prioritizing function over integration. Of course, new technologies were applied during the blockbuster era, but they aimed to fulfill a Pharma 2.0 business case and were not intended to be game changers.
Currently, the pharmaceutical industry is experiencing product differentiation (driven by rapid progress in the areas of biotech, genetics, and treatments for rare diseases) and market segmentation (driven by a larger, aging population and a global market with different regional opportunities and constraints). The industry needs a game changer: Pharma 4.0TM.
The section on Pharma 4.0™ operating model elements later in this article offers insights into this desired state. Whereas the elements and enablers of Industry 4.0 might generally apply to all industries, Pharma 4.0™ is distinguished by the need of the pharmaceutical industry and its regulators to safeguard patient safety and health outcomes. This priority requires the alignment of quality control with Pharma 4.0™; additionally, the control strategy must be empowered with the same operating model and technologies used in development and operations. In other words, a holistic control strategy is needed.
At the dawn of the transition from Pharma 2.0 to Pharma 4.0™, the following hurdles have become apparent:
- The industry is in a hybrid period in which blockbusters and niche products coexist.
- The prevailing Pharma 2.0 culture makes adopting changes difficult.
- The industry’s many management layers resist restructuring and adoption of new styles.
Two prerequisites are needed to overcome these hurdles:
- A strong drive and commitment from senior management to fulfill management responsibilities as defined in the pharmaceutical quality system (PQS) in ICH Q10
- A coalition of innovators and regulators to design acceptable roadmaps for the implementation of Pharma 4.0™
Defining Pharma 4.0™ Holistic Control Strategy
ICH Q10 defines “control strategy” as follows:1
A control strategy is a planned set of controls, derived from current product and process understanding, that assures process performance and product quality. ... Every drug substance manufacturing process, whether developed through a traditional or an enhanced approach (or some combination thereof), has an associated control strategy.
The ICH definition of the control strategy in ICH Q8, Q10, and Q111, 2, 3 is the basis for the description of the product, such as the quality target product profile (QTPP). Control of product and process are the main focus.
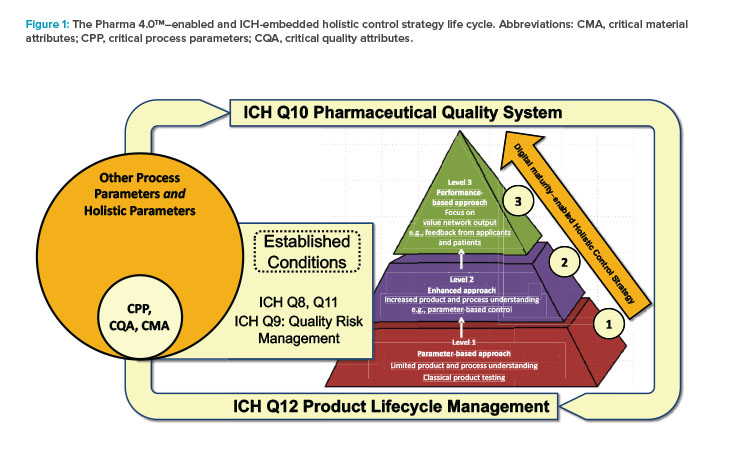
The Pharma 4.0™ term “holistic control strategy” is derived from this ICH definition. The term refers to an integrated approach to product and process life life-cycle management and ongoing change management (see Figure 1). This approach covers both the development chain and commercial manufacturing. Thus, the holistic control strategy targets all stakeholders, from the marketing and manufacturing authorization holders to the patient, including all supply chain parties. The holistic control strategy is enabled by digitalization as the necessary data are managed in real time, fully transparent, and available for sound real-time decision-making, improving quality and manufacturing process efficiency and accuracy. In sum, the holistic control strategy ties regulators, industry members, and patients together in an overall holistic value network structure driven by the Pharma 4.0™ operating model.
ICH guidelines Q8, Q10, Q11, and Q121, 2, 3, 4describe the framework for continuous improvement and product maintenance and product stewardship as part of ongoing ICH Q9–based quality risk management.5 This is an iterative approach applied throughout the entire life cycle of a product. The holistic approach includes parameters that are decoupled from process variability, going beyond the usual parameters considered during the development of a pharmaceutical product. Specifically, the holistic approach addresses all events that happen throughout the life cycle of the product, as well as the supply chain and patient needs and conditions.
In Figure 1, the authors have adapted the pyramid that the FDA6, 7, 8 uses to illustrate the concept of pharmaceutical quality to show how the main enabler of the Pharma 4.0™ concept, “digital maturity,” will scale across three levels:
- Level 1: Product testing–based control (e.g., classic quality control and in-process control testing)
- Level 2: Process-based control (e.g., process verification, automation, real-time release, parametric release, process analytical technology)
- Level 3: Performance- and patient outcome–based control (e.g., drug availability, drug efficacy, no adverse effects, convenience in application, support for patient compliance)
The method for applying the holistic control strategy depends on the maturity level of a pharmaceutical manufacturer and the market environment; it should also reflect the manufacturer’s desired level of aspiration.
Pharma 4.0™ Operating Model Components
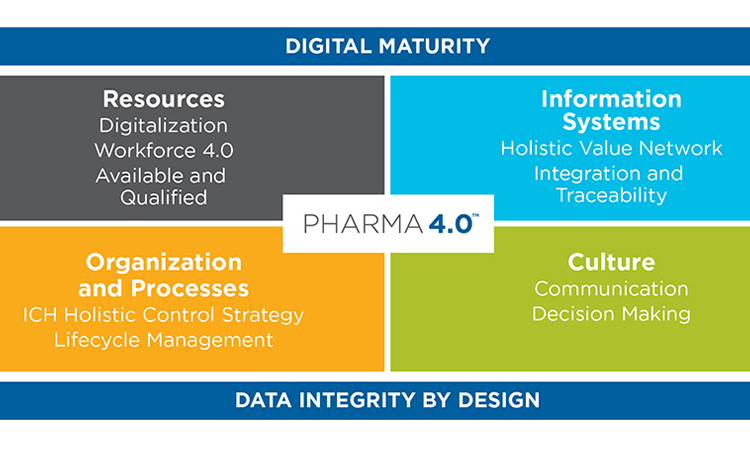
In 2017, the Pharmaceutical Engineering® article “A Holistic Approach to Production Control”9 introduced a model that extends ICH Q10 by adding four new elements (resources, information systems, organizations and processes, and culture) and two new enablers (digital maturity and data integrity by design). Full understanding of the holistic control strategy requires knowledge of these six building blocks, which have become the backbone of the ISPE Pharma 4.0™ operating model (Figure 2).
Resources
Resources are defined as an organization’s tangible resources, including its workforce (human resources); its machinery, equipment, and tools (physical resources); and the final product.
In Pharma 4.0™, resources are highly adaptive:
- Human resources are distinguished by cognitive flexibility, critical thinking, and creativity, and they require less control by governing bodies and formalized rules. Many recurring decisions are automated.
- Physical resources are designed to process very small series without extensive setup time. Examples of such resources are modular equipment, robotics, and three-dimensional printing.
- The human–machine interface is essential for adaptivity in a hybrid environment of human and physical resources. Virtual, augmented, and mixed reality will enable seamless collaboration during predicted and unpredicted events. A term often used for such a virtualized working environment is “digital twin”.10
Information Systems
Information systems are socio-technical systems in which information is provided based on economic criteria by both people and information and communication technology. These systems prepare, process, store, and transfer data and information.
Information systems provide one-, two-, and three-dimensional (1D, 2D, and 3D) integration of data:
- 1D integration means the aggregation of information into the decision hierarchy
- 2D integration means the communication of information along the value chain
- 3D integration means the communication of information across the value network (new in Industry 4.0)
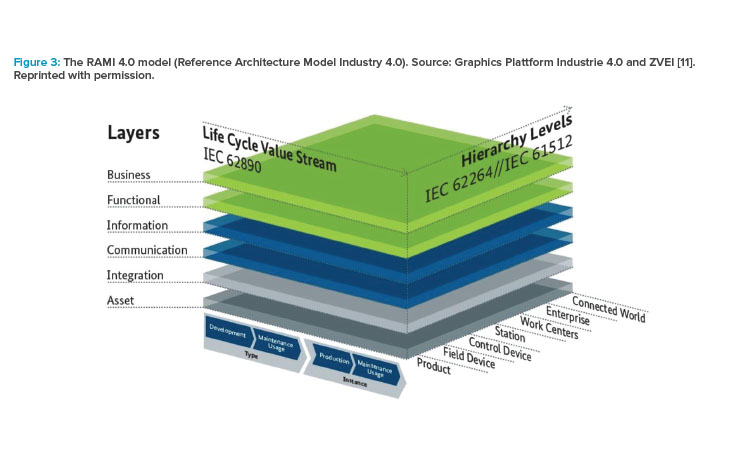
Information exchange between value chains that on their own drive toward different deliverables requires a move from a systems-based architecture to a service-oriented architecture. One example of 3D integration is the Reference Architecture Model Industry 4.0 (RAMI 4.0) architecture, which foresees a layered information structure in which data are the master and systems become services that allow continuous updating to adapt to new business requirements (Figure 3).11
Organization and Processes
Organizational structure refers to both a company’s internal organization (structure and operational processes) and the company’s position within the value network. It establishes mandatory rules that organize collaboration both within the company and externally.
In Pharma 4.0™, empowered value-driven organizations work in Agile processes (Figure 4). Employees are empowered with information that allows them to make operational decisions without a decision hierarchy. Transparency in the value network leaves no doubt that these decisions position the objectives of the network over the interests of an individual silo. At the same time, Agile processes prioritize achieving an early outcome of the right quality and aim to avoid extensive documentation.
Culture
Culture covers the value system within the company and thus describes the “soft” factors of collaboration. Organizational structure and culture are interdependent areas and must be cohesive.
The driving force behind culture is social conformity. When newcomers join an organization, it is mind-boggling how quickly they start conforming to that organization’s unwritten cultural expectations. Because cultural “rules” are informal, managers often feel that culture is not (completely) in their control. However, each industry-level transition has required cultural shifts; companies whose cultures do not change may likely fail to move forward.
In Pharma 4.0™, people no longer work in a single departmental silo; instead, they interact with different virtual communities, each having their own unwritten cultural norms. Because information exchanges are faster and more accurate than in the past, individuals working close to operations are capable of making decisions without waiting for authorization from others with more seniority or a higher position in the organizational hierarchy.
Digital Maturity
Digital maturity is the stepwise evolution of a current operating model into a Pharma 4.0™ operating model. To move up the digital maturity scale, organizations use an approach that is stepwise in two ways:
- It defines their start and finish points in a maturity index.
- It chooses the process or subprocess that creates a tangible outcome.
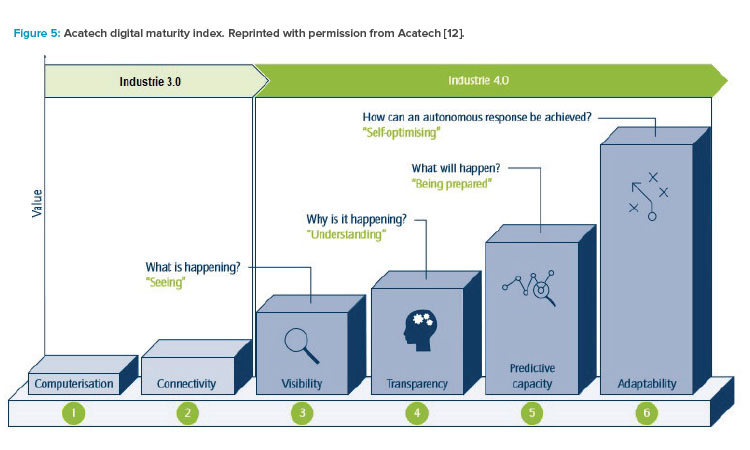
Digital maturity steps can be defined in a maturity index. By leveraging the same index, organizations can learn from other organizations’ experiences regarding challenges and outcomes. Within ISPE, the Pharma 4.0™ Special Interest Group adopted the Acatech maturity index to also leverage experiences from other industries,12, 13
- Level 1 of this index is the journey from “paper-based” to data-based operations. “Paper-based” means that electronic files not hosted by database systems do not have the same integrity as “wet ink on paper-based” systems.
- Level 2 is the step from expert systems to integrated systems (i.e., systems integration or service-oriented architecture).
- Level 3 is the 2D integration that allows visibility of information along the value chain. Organizations can see in real time what is happening.
- Level 4 uses tools and experience to analyze trends in level 3. It creates transparency about why things are happening and unravels the true root causes.
- Level 5 renders level 4 into plannability. Digital systems can predict what will happen, which allows organizations to be prepared.
- In Level 6, automated response technologies adapt processes to reliably generate the right outcome without manual intervention and despite changing input and circumstances.
Figure 512 illustrates the maturity of digital operations in Industry 3.0 and 4.0 operating models. Not every organization needs to move to these models. Moving up the scale can improve a company’s performance, but the incentive to move up is largely driven by market forces. For example, an exclusive watch or car manufacturer might not even need to move to Industry 2.0. Similarly, pharmaceutical companies that produce blockbuster products might not have sufficient incentive to adapt the Pharma 4.0™ model. On the other hand, companies moving to niche products or advanced therapy medicinal products might find that Pharma 4.0™ is the only way to sustain their operations.
Data Integrity by Design
Data integrity by design is the information architecture and prospective control of data quality within predefined organizational boundaries.
In Pharma 4.0TM, computerized systems are continuously updated and use enormous volumes of data to drive the organization’s decision-making. Regulators have already recognized that the focus in the information world is changing from application integrity to data integrity. This shift in focus is enforced at this very moment. Presently, stakeholders are retrospectively verifying data integrity in the existing landscape. The next step will be to prospectively design a data and systems architecture in which data integrity is intrinsic and data reside in platforms of collaborative data, where agreed-upon rules apply.
In such a highly versatile environment, it is impossible to sustain the same validation procedures that were used in Pharma 2.0 and 3.0. New computer system validation procedures must be designed to enable rapid life cycles for computer applications using data in platforms of proven data integrity.
Stakes and Stakeholders
Successful industry adoption of the holistic control strategy will involve many stakeholders, including regulators, senior management, standards-setting organizations, and patients.
Regulators
The holistic control strategy requires common understanding and alignment between industry and regulators. In general, to encourage investments in products and process transparency, regulators must shorten approval times for new drugs and for submission of changes and amendments. If the regulatory process is cumbersome, companies will be disinclined to invest in a holistic control strategy.
A holistic control strategy based on a completely digitalized value network has the potential to enable regulators to focus regulatory oversight and shorten product change management approval times. Therefore, the Pharma 4.0TM Special Interest Group recommends that industry offer training and education for regulators in the context of a holistic control strategy.
Senior Management
The application of a holistic control strategy offers to the management of a drug manufacturer a new balance between being transparent and being regulated. This can lead to faster approval times for submissions and help senior management fulfill their explicitly required “management responsibilities” as defined in the ICH Q10 pharmaceutical quality system.
Industry leaders and regulators should acknowledge that the proposed approach is not about camaraderie or companionship. Clear rule-setting can help maintain appropriate boundaries in the relationships between companies and regulators.
To ensure transparency, companies and management must get rid of functional silos and replace hierarchies with empowered cross-functional teams operating within value-driven organizations.
Standards-Setting Organizations
A holistic control strategy can only be effective if regulatory authorities agree to uphold common technical and regulatory standards. Trade wars, trade barriers, and unharmonized systems are the enemies of progress.
Standards-setting organizations such as PIC/S and ICH can develop frameworks for a holistic control strategy enabled by digitalized and integrated systems. Additionally, interagency initiatives by national health authorities such as the US FDA or European Medicines Agency, together with national competent authorities in the European community, could establish respected frameworks on a smaller scale.
Social media can play a major role in influencing key stakeholders to agree to develop common technical standards as business enablers.
A holistic control strategy promises to create more industry transparency and better oversight for patients and consumers as well as regulators.
Patients and Consumers
The roles of patients and consumers in disease prevention and management are rapidly expanding as individuals are using digital tools to connect with other stakeholders in the healthcare system. Notably, a holistic control strategy promises to create more industry transparency and better oversight for patients and consumers as well as regulators. Patients are already seeking transparency in product pricing. In the future, patients may also directly de-mand that companies share data about, for example, medication risks; the reliability of quality and production; quality, safety, and efficacy rankings; and benchmark comparisons of products and companies. Statistics in connection with field alerts and recalls will be public as well, and patients will be able to use end-user devices to verify the authenticity of medicines, and much more.
Case Studies
Merck Healthcare KGaA: Augmented Reality
The biopharma industry is faced with increasing complexity in terms of new manufacturing technologies, portfolio delivery, health authority regulatory requirements, and cost pressure. Therefore, digitalization is a critical element for the future of the biopharma industry (and other industries). With that in mind, the healthcare business sector at Merck Healthcare KGaA, in Darmstadt, Germany, and Mollet del Vallès, Spain, has begun its digital journey to cover the entire value chain. In manufacturing, augmented reality initiatives were kicked off in 2019 across the whole network, including at the state-of-the-art pharma packaging center in Darmstadt, to address some of these challenges.
Augmented reality is a great add-on tool for complex processes to help employees manage the daily operations in a standardized mechanism. It is used in the pharmaceutical industry in digital operations where accurate and paperless processes are deployed. At the Merck facilities, it is being used in devices such as hands-free smart glasses and mounted tablets with heads-up display interfaces and voice or touch controls (Figures 6 and 7).


The purpose of using augmented reality within Merck’s facilities and operations is to bring the right information to the right employee at the right time across multiple device types, and to foster proactive decision-making. Augmented reality will help overcome variability in operations. Variability is known to be reliability’s first enemy, and by using augmented reality tools, Merck expects to improve reliability; specifically, the main augmented reality functionalities affect quality, safety, production, personnel, and data.
Implementing and deploying augmented reality to reduce variability accelerates the pace of operations at Merck Healthcare KGaA in Darmstadt. Augmented reality can be used in several applications such as changeovers, maintenance, line clearance, cleaning activities, and training. It will increase the quality of operations significantly, save time, ensure a reliable quality of the tasks, support on-time technical problem-solving, and increase productivity and efficiency to deliver positive results. Additionally, the data produced from the augmented reality applications can be used to continuously improve processes.
Overall, an augmented reality system in the manufacturing area is a digital driver for quality, efficiency, and standardization. Augmented reality enables employees to master increasingly complex machines and workflows.
Sanofi: Applying BITMAP
Recently, the challenge for organizations of managing knowledge efficiently and effectively has noticeably increased due to the volume of data, information, and knowledge as well as the pace at which data are produced. Also, organizations may learn at a slower speed than is potentially possible be-cause tacit knowledge is difficult to capture and make accessible to everyone who needs it.
Recognizing that this situation influences how innovation is managed, Sanofi decided to experiment with new ways for people to connect and share and access knowledge. The goal is to leverage existing internal and external knowledge, connect experts internationally, and improve the creation of both incremental and breakthrough ideas related to manufacturing processes in research and development and industrial affairs.
In the age of Industry 4.0, digital technologies become increasing important for managing the amount of data and information produced in an organization, and for using advanced analytics (e.g., machine learning) to create knowledge that makes us smarter. For example, digitalization helps predict manufacturing processes, assess the impact of manufacturing process parameters on product quality and quantity, and, ultimately, provide a holistic view and understanding of how those parameters influence each other during the life cycle of a drug. Digitalization can also help with company-wide sharing of information as people use digitized workflows to perform daily activities and routines.
Biologics Innovation and Technology Management Process (BITMAP) is a concrete example of how Sanofi has used digital technologies to help in the field of knowledge management. BITMAP is composed of three elements: people, digitized business processes/workflows, and a smart IT platform.
Sanofi is a global organization. When a worker is interested in how the company is addressing a specific issue, that worker might have no idea who to contact. Biologics Innovation and Technology Management Process connects people across the company to all topics that are internally addressed. A smart search engine and digitized workflows have thus helped Sanofi employees to be more efficient and effective in managing and exchanging knowledge and ultimately enable innovation for manufacturing technologies in biologics.