With the start of the new year, Pharmaceutical Engineering® is launching a new series of profiles of industry leaders. This ongoing series will look at the lives and careers of individuals who are changing the face of the pharmaceutical industry.

Downloads
Introducing Industry Leaders:
Pharmaceutical Engineering® is launching a new series of profiles of industry leaders. This ongoing series will look at the lives and careers of individuals who are changing the face of the pharmaceutical industry.
An Advocate for Quality: Ranjana B. Pathak
Ranjana Pathak has spent nearly 40 years in the pharmaceutical industry. Currently the President and Global Head of Quality, Medical affairs, and Pharmacovigilance at Cipla Ltd. in Mumbai, India, Pathak’s long tenure has afforded her an informed perspective on the past, present, and future of the industry.
Bringing Pharma 4.0TM into the World: Christian Wölbeling
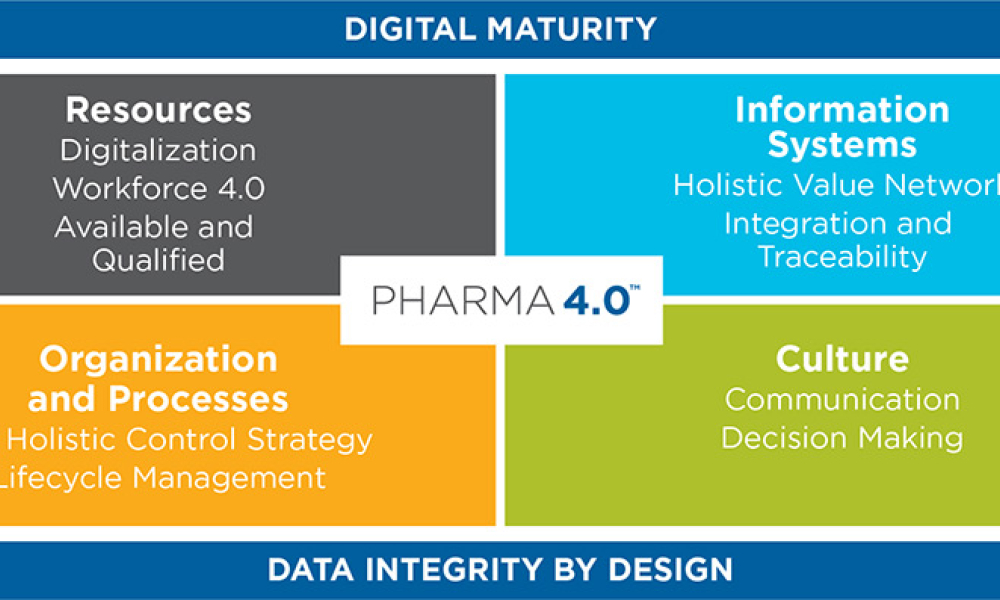
The pharma industry has its Pharma 4.0TM champion in Christian Wölbeling, an IT expert. Starting with ideas jotted down on a restaurant napkin, he has tirelessly and expertly led ISPE’s global efforts to define the industry’s slow but sure progression toward a holistic strategy to achieve the Pharma 4.0TM goal.
In Search of Flexibility: The Biotech Industry’s Continuing Quest for Optimized Manufacturing Facility Design.
This article takes a historical look at the evolution of facility design, specifically focused on how “flexibility” has advanced to the industry’s current process and possible future states.
Applying Holistic Control Strategy in Pharma 4.0TM
The ISPE Pharma 4.0TM Special Interest Group has designed an operating model as a framework for how digitalization can be applied to ICH Q8–Q12 and named it the “holistic control strategy.” This article introduces the strategy and presents two case studies of companies that have started to apply it.
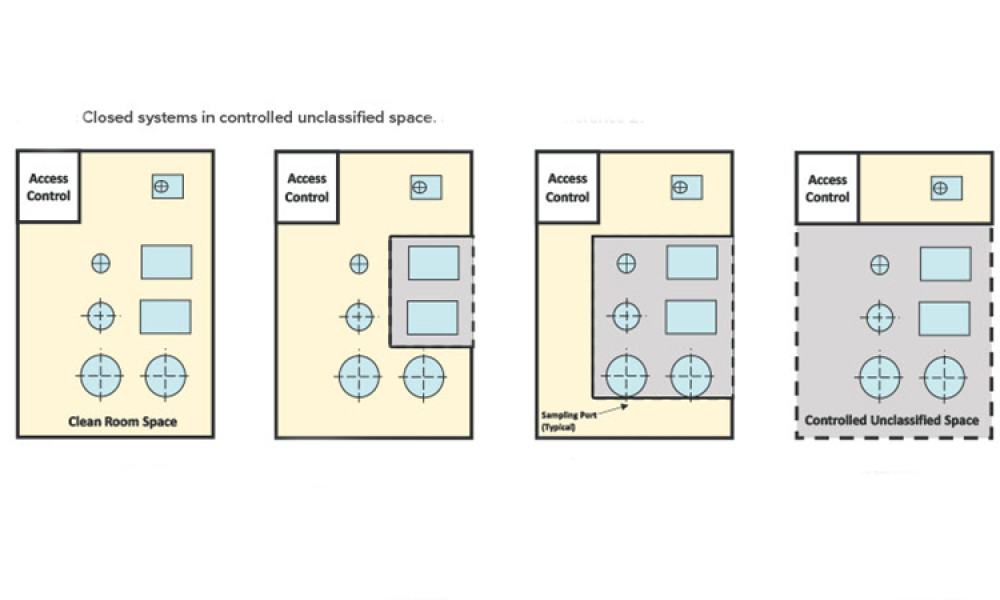
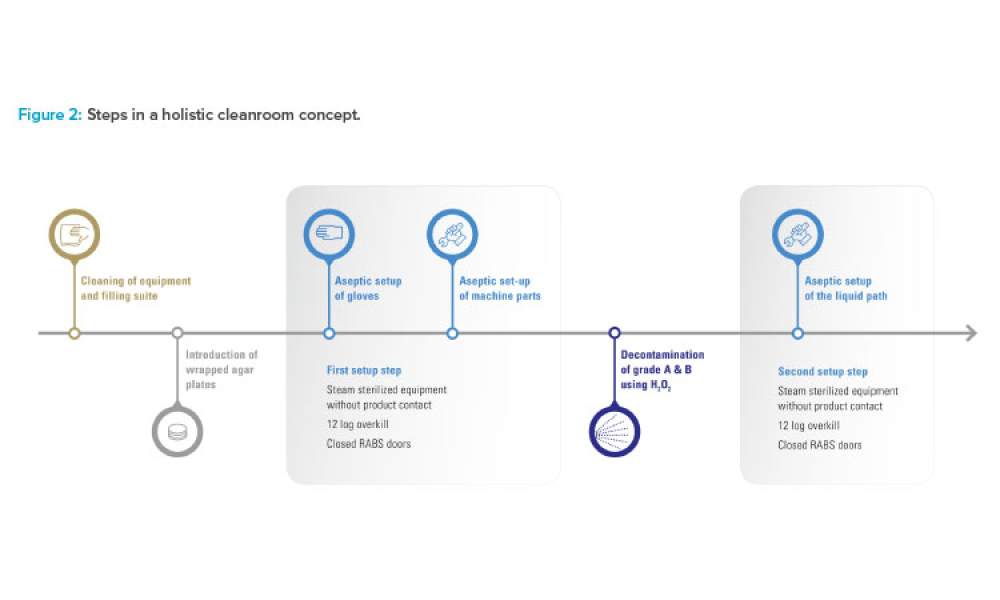
Industry Perspective: A Holistic Cleanroom Concept: Higher Quality and Greater Flexibility
Looking forward, manufacturers should anticipate future format, packaging, and filling needs, and seek technologies with sufficient built-in versatility. This article explores a promising holistic cleanroom concept for high-quality aseptic processing of drugs.
In This Issue
As I sit at my computer to write my first column as ISPE International Board Chair, my thoughts are centered on how I can personally help drive our unifying vision, “Connecting Pharmaceutical Knowledge,” and how we as members of ISPE have an outstanding opportunity in the year ahead to provide outreach and resources to expand our networks of people, technology, and information to accomplish...
The pharmaceutical industry is ever-changing, as we saw demonstrated during the 2019 ISPE Annual Meeting & Expo, and at other ISPE conferences around the world. In fact, change is accelerating—and to...
Ranjana B. Pathak, BSc (Hons), MBA, DHA, has spent nearly 40 years in the pharmaceutical industry. Currently the President and Global Head of Quality, Medical Affairs, and Pharmacovigilance at Cipla Ltd. in Mumbai, India, Pathak’s long tenure has afforded her an informed perspective on the past, present, and future of the industry. An active member of the
Every important cause needs its champion. Champions have a vision of how things should be, and a passion to reach their goals. They are committed and determined to achieve positive results, are willing to do the heavy lifting, and will take consistent and massive action until results are achieved.
Since the early 1990s, when the “upstart” biotech industry realized that its future success would be heavily influenced by the ability to manufacture multiple products within the same facility,1
Applying emerging technologies can lead to more robust and flexible manufacturing processes that in turn can help the pharmaceutical industry respond to drug shortages, reduce interruptions in production and delivery of medicines, ensure consistent clinical performance of products, and achieve other benefits. Although some may believe that regulators are averse to the use of emerging...
As the pharmaceutical industry balances demands for small-batch and blockbuster products and encounters new regulations, there is a need for efficient and safe production technologies that can meet stringent quality and safety requirements for the aseptic filling of drugs. Looking forward, manufacturers should anticipate future format, packaging, and filling needs, and seek technologies with...
Since its inception at the ISPE 2016 Annual Meeting & Expo, Women in Pharma® (WIP) has been rapidly growing. In the US and Europe, the society has held numerous Women in Pharma® events at local Affiliates, Chapters, and...
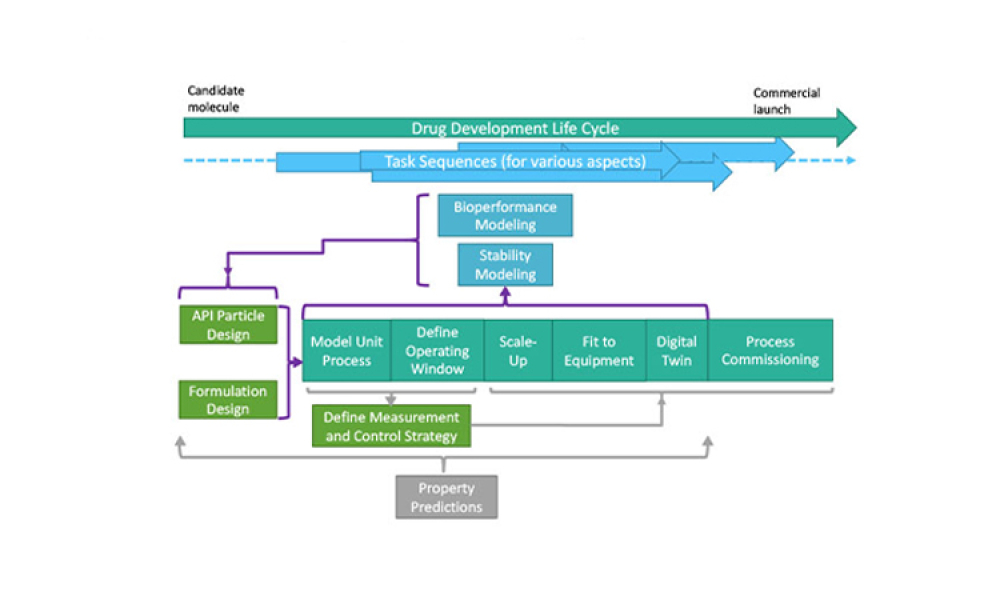
The Advanced Digital Design of Pharmaceutical Therapeutics (ADDoPT) project1 is a recently completed UK-based design manufacture and supply chain research collaboration. This collaboration catalyzed work to...
According to US Pharmacopeia (USP) Chapter <790>, “all parenteral products should be essentially free from any visible particles.”1 This is the first and foremost requirement stated in all pharmacopeia for any injectable product. However,...
ISPE’s online site for Pharmaceutical Engineering was launched a little over one year ago, in November 2018. Here are some statistics about the site’s success during its first year.
On 26–27 October 2019, the first Global Student & Young Professional Hackathon was held at the 2019 ISPE Annual Meeting & Expo. Thirty-six