Aseptic fill-and-finish processing faces special challenges. On the one hand, demand is increasing for small-batch products to treat rare diseases; on the other hand, blockbuster drugs require large batch sizes. To meet divergent product requirements, pharmaceutical companies need flexible manufacturing systems that enable individual line setup for product changeovers.
Additionally, new and revised guidelines updating global regulatory requirements for process safety are expected in the near future. For example, Annex 1 of the EU GMP Guide “Manufacture of Sterile Medicinal Products”—which is considered the most important European regulatory standard for the manufacturing of sterile pharmaceutical products—is under review and being updated. In its current draft version, Annex 1 states that the expected result of microbiological findings within isolators and restricted access barrier systems (RABSs) is 0 CFU recovered, and “all critical surfaces that come into contact with sterile materials should be sterile.” There is also a trend in visual inspection to increase requirements regarding detection of particles.
Conventional Technologies
To date, two technologies for high-quality aseptic processing of drugs have stood out: isolators and restricted access barrier systems. It is important to differentiate between these systems because they offer different types of product protection.
Isolators are completely sealed units, entirely “isolated” from the outside environment. They undergo extensive decontamination that results in constraint levels of adaptability and efficiency.
Restricted access barrier systems technology involves barrier and dynamic airflow separation between the environment and the drug product. Compared with isolators, restricted access barrier systems offer advantages of faster setup, efficient product changeover, and variability. As such, restricted access barrier systems are appropriate for manufacturing operations with several products and short downtimes.
A New Alternative
The starting point for the new technology described in this article was a passive open restricted access barrier systems with closed doors that provides a high level of aseptic control. Thus, the barrier surrounding a grade A clean area is open at the top and the bottom toward a grade B area and is supplied with laminar air from the ceiling of the cleanroom. The barrier can be opened for installation of huge format parts at the beginning of setup. During filling, interventions are only allowed through built-in gloves. Any opening during filling will result in immediate termination of the batch and the discarding of all open units within the barrier.
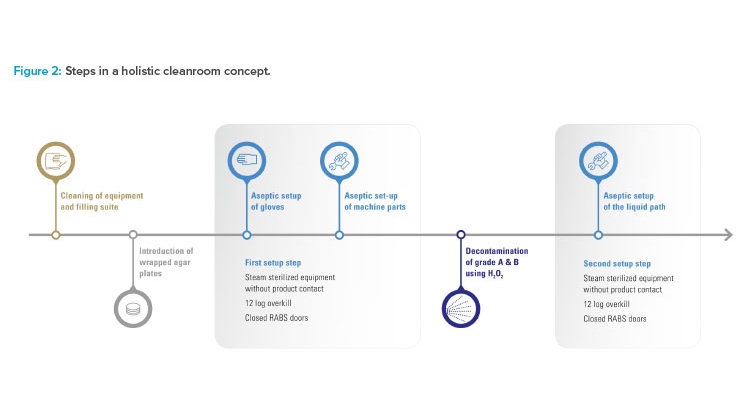
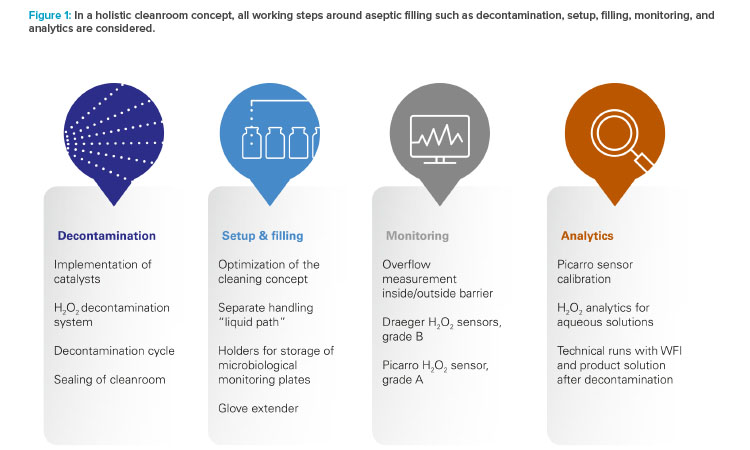
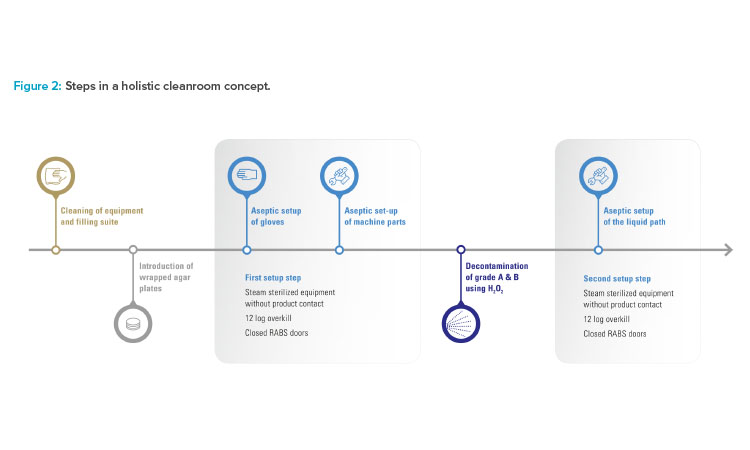
To improve the already high level of aseptic control, Vetter decided not to convert the existing restricted access barrier systems lines in isolator lines. Instead, the benefits of both conventional solutions were taken to create an alternative, Vetter Cleanroom Technology (V-CRT). Based on a passive open restricted access barrier systems, automated hydrogen peroxide (H₂O₂) decontamination, already well established within isolator technology, plays the most important role in this concept. Being a holistic concept, the technology is not limited to decontamination and its directly linked systems. It also includes setup and filling, and monitoring as well as analytics (Figure 1).
Decontamination
Decontamination is the core element of the holistic cleanroom concept. With automated H₂O₂ decontamination, unwieldy processes such as formaldehyde fumigation are replaced and sources of error in manual decontamination are minimized. Automated H₂O₂ decontamination also provides greater protection from microbiological contamination than wipe-and-spray disinfection. H₂O₂ is an effective decontaminant because it removes critical micro-organisms in grade A areas. In addition, it is practically residue-free because the solution quickly breaks down into water and oxygen.