Temperature & Humidity Requirements in Pharmaceutical Facilities
![Figure 1: Seasonal comfort (temperature and RH). ©ASHRAE, www.ashrae.org. Used with permission from 2017 ANSI/ASHRAE Standard 55 [4].](/sites/default/files/styles/site_750_x_420/public/2021-08/0921_PE_SO_TechHaycock_01.jpg?itok=xzCGKQvF)
Defining room temperature and humidity limits is a frequent topic of debate when designing and operating pharmaceutical and biotechnology facilities. What are appropriate alarm limits and acceptable durations for an alarm condition? Understanding the source of temperature and humidity requirements, and strategies for setting limits, can ensure both compliance and optimum use of energy. This article provides guidance on these topics, with supporting rationales.
Although temperature and humidity are both understood to be critical factors in the design and operation of compliant manufacturing facilities, regulations on these topics are surprisingly vague. In general, regulations suggest that temperature and humidity should be appropriate within manufacturing areas, with attention given to long-term storage areas, although needs are understood to be largely product specific. Tables 1 and 2 present key EU and US regulatory guidance from EudraLex1,2 and the US Code of Federal Regulations (CFR),3 respectively.
Considerations When Specifying Design Ranges
If there are no temperature or humidity limits for the product/process (which is often the case in closed processes with “internal” environmental controls), the temperature control of surrounding spaces defaults to that which is required for human comfort and to minimize shedding. An approach that ensures facility conditions are appropriate for the product and process, as well as for the gowning level of the workers, forms a reasonable basis for establishing the conditions for cGMP facilities.
| Source | Regulatory Guidance |
|---|---|
| EudraLex Volume 4, Part 1, Chapter 3 | 3.3. Lighting, temperature, humidity and ventilation should be appropriate and such that they do not adversely affect, directly or indirectly, either the medicinal products during their manufacture and storage, or the accurate functioning of equipment. 3.19. Storage areas should be designed or adapted to ensure good storage conditions. In particular, they should be clean and dry and maintained within acceptable temperature limits. Where special storage conditions are required (e.g. temperature, humidity) these should be provided, checked and monitored. |
| EudraLex Volume 4, Annex 1 | 16. Other characteristics such as temperature and relative humidity depend on the product and nature of the operations carried out. These parameters should not interfere with the defined cleanliness standard. 73. Activities in clean areas and especially when aseptic operations are in progress should be kept to a minimum and movement of personnel should be controlled and methodical, to avoid excessive shedding of particles and organisms due to over-vigorous activity. The ambient temperature and humidity should not be uncomfortably high because of the nature of the garments worn.a |
aNote that this guidance indicates that temperature may be governed by factors other than product or process requirements, such as the level of gowning.
| CFR Section | Regulatory Guidance |
|---|---|
| 211.42.3.10 | Operations shall be performed within specifically defined areas of adequate size. There shall be separate or defined areas or such other control systems for the fi rm’s operations as are necessary to prevent contamination or mix-ups during the course of the following procedures…Aseptic processing, which includes as appropriate…(ii) Temperature and humidity controls. |
| 211.46b | Equipment for adequate control over air pressure, microorganisms, dust, humidity, and temperature shall be provided when appropriate for the manufacture, processing, packing, or holding of a drug product. |
| 211.142 | Warehousing procedures. Written procedures describing the warehousing of drug products shall be established and followed. (b) Storage of drug products under appropriate conditions of temperature, humidity, and light so that the identity, strength, quality, and purity of the drug products are not affected. |
![Figure 1: Seasonal comfort (temperature and RH). ©ASHRAE, www.ashrae.org. Used with permission from 2017 ANSI/ASHRAE Standard 55 [4].](/sites/default/files/2021-08/0921_PE_SO_TechHaycock_01_0.jpg)
Comfortable Conditions
Good engineering practice suggests specifying conditions that provide comfortable conditions for most workers. Factors to consider when defining comfortable conditions include:
- Metabolic rate (activity level)
- Amount of clothing
- Temperature
- Local airspeed
- Humidity
- Skin state (wetness)
- Individual preferences
There are several methods for defining the conditions that most people will find comfortable. For example, in the US, ANSI/ASHRAE Standard 55 Thermal Environmental Conditions for Human Occupancy4 establishes a range of indoor conditions (temperature and relative humidity [RH]) intended to provide acceptable thermal comfort for building occupants (Figure 1). This American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) standard was first published in 1996 and has been regularly updated since 2004, with the most recent version published in 2017. The Chartered Institute of Building Services Engineers in the UK produces similar guidance.5
The comfort levels indicated in Figure 1 assume the building occupant is wearing “street clothes” without overgarments. The addition of gowning would drive acceptable temperature and humidity levels down (in order to maintain a consistent RH). Common industry practice is to provide lower environmental temperatures as the level of gowning increases. We suggest that room temperatures for occupants wearing “street clothes” should have a setpoint around 22°C, whereas ISO 8 environments should have a setpoint around 20°C and ISO 7 environments a setpoint closer to 17°C–18°C.
These suggestions are based on the need for higher levels of gowning in more highly classified areas (Grade A or B,2 or ISO 5 or 7, in operation), which may include plant uniforms, coveralls, hoods, overboots, sleeves, and double gloves. In these situations, it is usual to set the lower end of temperature acceptance criteria quite low (e.g., 10°C). When designing internationally, it is important to also consider local customs and practices, as these may impact workers’ comfort.
Microbial Growth
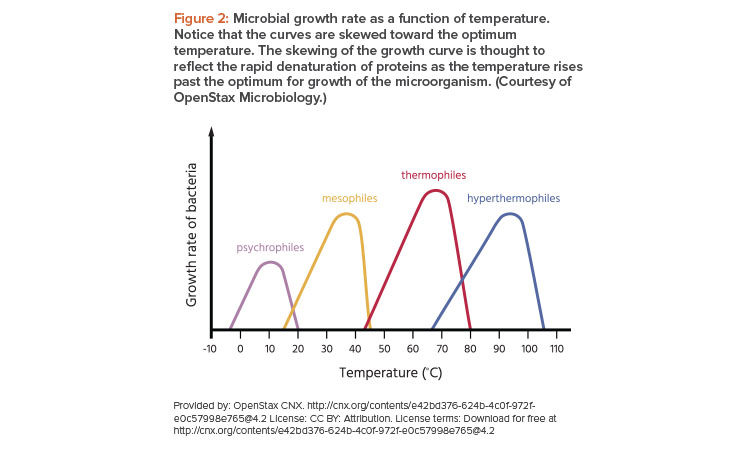
It is well known that temperature and humidity impact microbial growth and germination of spores (Figure 2).6
Temperature is a consideration for microbial growth: An increase in room temperature from 20°C to 25°C can roughly double the rate of bacterial multiplication. Mold propagation is more likely at warmer temperatures (up to about 35°C–40°C). Humidity also has an impact on the ability of the environment to support growth of any viable contaminants.
Water Activity
The ISPE Good Practice Guide: Process Gases7 discusses water activity in relation to microbial and sporicidal growth. This guidance is based on USP<1112>: Application of Water Activity Determination to Nonsterile Pharmaceutical Products,8 which states:
Water activity, aW, is the ratio of vapor pressure of H2O in product (P) to vapor pressure of pure H2O (Po) at the same temperature. It is numerically equal to 1/100 of the relative humidity (RH) generated by the product in a closed system. RH can be calculated from direct measurements of partial vapor pressure or dew point or indirect measurement by sensors whose physical or electric characteristics are altered by the RH to which they are exposed.
The relationship between aW and equilibrium relative humidity (ERH) is represented by the following equations:
aW = P/Po and ERH (%) = aW × 100
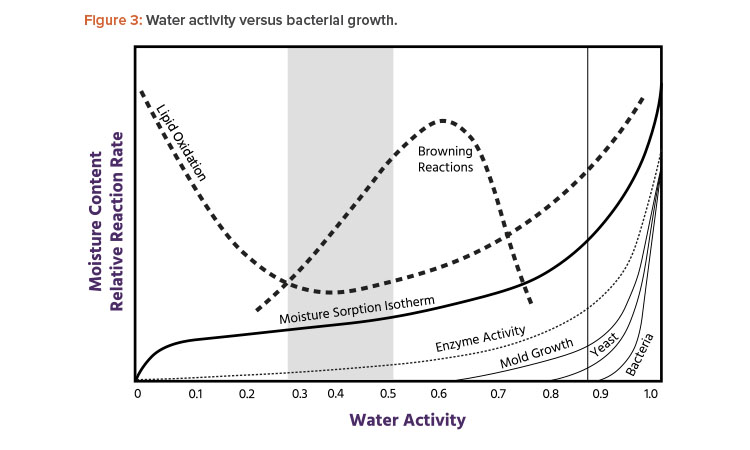
Microbial growth and germination of spores are very unlikely in areas with an RH below 60% (see Figure 3). Figure 4 shows the relationship between environmental health and comfort.9
Vegetative cells would normally be carried on particles, such as skin flakes, and are fragile. Mold spores and bacterial spores are much hardier; they can exist as individual spores and then grow when they find a suitable growth environment. They can enter a facility on people or materials.
Bacterial spores are typically 1–2 μm in length and 0.5–1.0 μm in diameter. Mold spores are typically larger, between 4 and 20 microns.10 Mycotoxins are toxins produced by some species of mold (secondary metabolites) and are as small as 0.1 μm. Aspergillus species and Penicillium species mold spores range from 1 to 8 μm.
Keep in mind that although high-efficiency particulate air (HEPA) and ultra-low particulate air (ULPA) filters are highly effective against microbial contamination, they are not truly absolute filters. If the microbial load upstream of these filters is high enough, some penetration is possible.
![Figure 4: Environmental health and comfort. Reprinted from [9].](/sites/default/files/2021-08/0921_PE_SO_TechHaycock_04.jpg)
Storage Spaces
According to USP<659>: Packaging and Storage Requirements,11 temperature and humidity conditions for the acceptable storage of materials are divided into freezer, refrigerator, cold, cool, controlled room temperature (CRT), warm, and excessive heat. With regard to temperature and storage, USP<659> further states:
Specific directions are stated in some monographs with respect to storage conditions (e.g., the temperature or humidity) at which an article must be stored and shipped. Such directions apply except where the label on the article has different storage conditions that are based on stability studies. Where no specific directions or limitations are provided in the article’s labelling, articles must be protected from moisture, freezing, and excessive heat, and, where necessary, from light during shipping and distribution. Drug substances are exempt from this standard.
Based on this guidance, we understand that the “ambient storage” conditions used for ambient storage warehouses should be aligned with the USP CRT guidance, which states, “Storage spaces classified as ‘ambient’ are therefore expected to be not more than 25°C on average with a 15–30°C acceptance range.”11
USP<659> defines CRT as follows:11
The temperature maintained thermostatically that encompasses the usual and customary working environment of 20°–25° (68°–77°F). The following conditions also apply. Mean kinetic temperature not to exceed 25°. Excursions between 15° and 30° (59° and 86°F) that are experienced in pharmacies, hospitals, and warehouses, and during shipping are allowed. Provided the mean kinetic temperature does not exceed 25°, transient spikes up to 40° are permitted as long as they do not exceed 24 h. Spikes above 40° may be permitted only if the manufacturer so instructs.
Articles may be labeled for storage at “controlled room temperature” or at “20°–25°”, or other wording based on the same mean kinetic temperature….
An article for which storage at Controlled room temperature is directed may, alternatively, be stored and shipped in a cool place or refrigerated, unless otherwise specified in the individual monograph or on the label.
Storage spaces classified as “ambient” are therefore expected to be not more than 25°C on average, with a 15°C–30°C acceptance range.
Temperature Range Recommendations
CRT Storage Spaces
The US Pharmacopeia uses the mean kinetic temperature (MKT, ”the single calculated temperature at which the total amount of degradation over a particular period is equal to the sum of the individual degradations that would occur at various temperatures”) in its CRT guidance.11 However, it is common practice to set “engineering” (alert) alarms for CRT storage spaces at 20°C and 25°C with “quality” (action) alarms for these spaces at 15°C and 30°C. Keep in mind that the wider the used operational range is, the lower the energy consumption for heating, cooling, humidification, and dehumidification will be.
| Space | Lower Limit, °C | Operating Range, °C | Upper Limit, °C |
|---|---|---|---|
| Plant utilities room | 5 | 15–27 | 90 |
| Clean utilities (technical space) | 5 | 20–25 | 65 |
| Raw materials warehouse (ambient) | 10 | 15–30 | 35 |
| Raw materials warehouse | 15 | 20–25 | 30 |
| CNC | 15 | 20–24 | 30 |
| CNC+/Grade D | 15 | 18–22 | 30 |
| ISO 8/Grade C | 15 | 18–22 | 30 |
| ISO 7/Grade B | 14 | 17–20 | 30 |
| ISO 5/Grade A | 14 | 17–20 | 30 |
| Finished goods (refrigerated) | 2 | 3–7 | 8 |
| Finished goods (CRT) | 15 | 20–25 | 30 |
aAlert alarms should be set close to the operating limits to provide early notification of a potential issue; the limits given are proposed for initial settings used while gathering operational data.
Manufacturing Areas
Temperature is generally controlled in the range of 15°C–25°C for manufacturing and storage facilities, except where a process or product requires more stringent control. In the case of human comfort, the acceptable range may be broad (e.g., 20°C ± 5°C). However, general practice is to maintain control within a narrower range, such as ±2°C–3°C, and, in some cases (for example, for older control systems that may not be as responsive for temperature control), it is advisable to use a range that allows the temperature to heat up to a maximum temperature and cool down to a minimum temperature. It is also a good practice in terms of minimizing energy consumption to shift the setpoint seasonally to accommodate how workers’ perceptions of comfort shift with seasonal changes (see Figure 1).
Where temperature limits are defined by process or product requirements, industry best practice is to ensure that the alert range accounts for instrument error. The National Environmental Balancing Board suggests that instrument error should be within 1.0% of reading.12 This should be considered when setting alarm set points.
Ideally, alarm levels are determined after observing the variability of specific systems. Typically, a short alarm time delay is incorporated to avoid any nuisance alarms set off by short-term fluctuations that may be caused by an influx of air from an adjacent area that is operating at different conditions, or any signal noise.
Design Conditions
It is common for design conditions to be largely based around comfort factors (except where special conditions outside of these are needed for products or processes). Therefore, short-term excursions are often not a quality concern. Table 3 summarizes suggested temperature ranges to use as design conditions for various spaces, including controlled not classified (CNC) spaces. CNC spaces are:13
Areas where HVAC systems are specifically designed to reduce airborne contaminants below the level of the ambient environment and both temperature and RH are controlled more tightly than in the ambient environment. Claims for environmental control in these areas are related to the design of the system; installation qualification is common. No claim is made or qualified for the specific control of particulate. Typical systems will have heating, cooling, and filtration meeting minimum efficiency reporting values of 13 (F7) or better. These areas are sometimes referred to as “pharmaceutical” or “clean” areas within pharmaceutical facilities.
The temperature values presented are lower than suggested in the ISPE Baseline Guide, Volume 6: Biopharmaceutical Manufacturing Facilities, second edition,14 based on our experience of operator comfort in the gowning associated with each classification of space.
Humidity Range Recommendations
Design and Operating Ranges
As with temperature, regulatory guidance suggests that humidity levels should be appropriate for the product and process as well as for operator comfort and to limit shedding and sweating. Therefore, if humidity does not affect product and process, RH is commonly maintained within a band, at levels less than 60% (see Figure 4), with the lower RH limit defined by local custom and practice. Controlling the lower range of humidity is not generally important to address microorganism-related concerns, but it may be important to manage static electricity, human comfort, and shedding.
The acceptance range can be broad: It is not uncommon to see humidity acceptance ranges between 30% and 60% RH, as suggested by ASHRAE4 (see Figure 4) and the ISPE Baseline Guide.14 Some companies successfully extend the acceptance range from 20% to 70% RH, based on the location (with consideration of local flora and fauna, cleaning methods, etc.).
To achieve the acceptance range, general practice is to maintain setpoints near 25%–55% RH and allow the system to respond if RH levels exceed those limits. These limits may be adjusted seasonally to accommodate how workers’ perceptions of comfort shift with seasonal changes (see Figure 1).
| Space | Lower Limit, % RH | Operating Range, % RH | Upper Limit, % RH |
|---|---|---|---|
| Plant utilities room | N/A | ≤80 | 90 |
| Clean utilities (technical space) | N/A | ≤60 | 65b |
| Raw materials warehouse (ambient) | N/A | 20–80 | 90 |
| Raw materials warehouse (controlled) | N/A | 30–60 | 65b |
| CNC | 25 | 30–60 | 65b |
| CNC+/Grade D | 25 | 30–60 | 65b |
| ISO 8/Grade C | 25 | 30–60 | 65b |
| ISO 7/Grade B | 25 | 30–60 | 65b |
| ISO 5/Grade A | 25 | 30–60 | 65b |
| Finished goods (refrigerated) | N/A | 20–80 | 90 |
| Finished goods (CRT) | N/A | 20–75 | 80 |
| Dry storage (CRT) | N/A | 30–40 | 45 |
aBroader operating ranges with higher upper limits have been successfully used with appropriate qualification and monitoring of data regarding the impact of RH on local microbial growth. Alert alarms should be set close to the operating limits to provide early notification of a potential issue; the limits given in the table are proposed for initial settings used while gathering operational data. N/A = not applicable.
bUpper limits may be raised with proper qualification and data.
Storage Spaces
According to the US Pharmacopeia, humidity conditions for the acceptable storage of materials are divided into “dry” and unspecified conditions. The USP<659>11 definition of a “dry” place is as follows:
A place that does not exceed 40% average relative humidity at 20° (68°F) or the equivalent water vapor pressure at other temperatures. The determination may be made by direct measurement at the place. Determination is based on NLT [not less than] 12 equally spaced measurements that encompass either a season, a year, or, where recorded data demonstrate, the storage period of the article. There may be values of up to 45% relative humidity provided that the average value does not exceed 40% relative humidity. Storage in a Container validated to protect the article from moisture vapor, including storage in bulk, is considered a Dry place.
Humidity Alarm Set Points and Delay Times
It should be noted that, at a constant absolute humidity, RH increases as temperature decreases, making low-temperature environments more susceptible to exceeding RH limits. If moisture is a product or process requirement, it may be more efficient to control moisture to an absolute level (grams of water per kilogram of air) to remove the effect of temperature; often the humidity issue is due to the partial vapor pressure.
High RH levels can initiate a biological process. Because the biological processes of mold spore germination or bacterial reproduction have time constants of their own, it is normal to accept a delay in the initiation of RH alarms that is shorter than the time required for the biological process to move to completion, and longer than the time required for typical wet tasks, like cleaning. In the case of common mold types on building materials, the germination period often exceeds 24 hours. For this reason, although the speed of germination will likely increase with increases in RH, there is a period during which any RH level, even close to 100%, may be tolerated for short times. Common wet cleaning processes rely on this principle, as the water that is applied to a surface during cleaning will not promote mold propagation so long as it evaporates within a reasonable period. However, if an area is allowed to stay at a high humidity level for an extended period (e.g., in a shower), microorganisms can propagate.
Alarm set points should consider instrument error. The National Environmental Balancing Board suggests that verification instrument error should be within 2% of the reading range.12 Coupled with the measurement instrument error, this suggests that alert ranges should not normally be less than ±5%.
For these reasons, it is suggested that alarms for RH incorporate delays as needed to accommodate excursions caused by normal operation (doors opening, cleaning, etc.). A period of 1–4 hours is common for an alarm delay, given that this period is usually enough to complete cleaning but does not provide time for significant expansion of microbial colonies or germination of mold spores. Delays of up to 24 hours may be justified based on the time required for microbial contaminants to propagate in a region.
For existing facilities that are not designed to control RH to less than 65%, an alarm delay of 12 hours could be used for RH above 70%, prior to cessation of operations. Low-level alarm set points would be based on local practices.
Note that in all cases, vented steam or hot water vapor from component washing and equipment cleaning/sanitization should have a local exhaust.
Design Conditions
It is common for design conditions to be based around a combination of comfort factors and upper limits to inhibit microbial growth (except in situations where special conditions are needed for products or processes, such as effervescent tablet manufacturing). Again, short-term excursions are often not a quality concern. Table 4 summarizes suggested RH ranges to use as design conditions for various spaces.
Practical Advice for Controlling Mold
Fungal spores are always present. If the prevailing conditions do not encourage growth, there are no problems; however, many facilities experience mold issues. According to the Centers for Disease Control and Prevention, when buildings are wet for more than 48 hours (e.g., following a flood or hurricane), “visible and extensive mold growth” is likely.15
Mold problems may be identified by higher particle counts, followed by visible signs of contamination, or through environmental monitoring. According to an industry specialist in restoration, “under ideal conditions (optimal temperature and level of humidity), it takes 24 to 48 hours for mold to germinate and grow. Typically, the spores begin to colonize in 3 to 12 days and become visible in about 18–21 days.”16
For mold spores to germinate and proliferate, conditions must be conducive to growth. For example, a pipe leak could allow water to enter drywall (gypsum board) or cleanroom panels, or water used during cleaning could penetrate a joint to sit on timber. In a cold room or freezer, mold may grow when high humidity is caused by an internal air leak around a utility penetration, leading to internal condensation.
If mold is suspected or confirmed, the following points may be worthy of investigation:
- Residual moisture in ductwork: Common sources of moisture include damaged vapor barriers on ductwork insultation or the humidification system not draining fully.
- Residual moisture in and around air-handling units (AHUs): poorly draining condensate pans under cooling coils, poor sealing of insulated AHU panels, and air leakage from the negative pressure section can allow moisture and condensation.
- High room-humidity levels: Consider reducing the RH level to approximately 55%.
- Leakage of contaminated air: Determine whether airflow direction changed, even for a short time period. This issue can be caused by poor sealing between cleanroom panels. In one case, the process area HVAC was turned off to ensure there was adequate contact time for the sanitizing material, and this allowed contaminated air to leak in due to “loose” mastic seals between panes; the adjacent service area was at a slightly higher pressure, and there was a drain by the poor joint in the service area.
- Sanitization and cleaning practices: Are all cleaning materials, including mops, sanitized? Are the cleaning or sanitizing materials a potential source of contamination?
- Spore transfer from another area: For example, contamination could be due to an instrument tech wheeling a cart from one building to another, with inadequate wheel cleaning (dedicated carts are preferable). A worker’s shoes or clothing could also carry contaminants from one region to another.
- The adequacy of sealed cleanroom finishes: In one instance, mold was found on plywood blocking behind the wall finish, where the seal was inadequate. In another case, mold was found in a hole in the top of a door, which turned out to be stainless cladding over wood.
- Sources of condensation between walls due to temperature gradients in adjacent rooms: For example, if a wall in a cold room wall sweats, investigate whether the damp-proof membrane is in the right location.
- Equipment operating below the dewpoint of the conditioned space: This can result in the formation of condensation.
Prompt remediation is required when mold is discovered. For porous materials, cleaning or sanitizing is difficult, and replacing contaminated materials is advised. If drywall is contaminated, the only solution is demolition and replacement. For nonporous materials, cleaning and sanitizing can be effective once the source of moisture is removed.





