Industry Leaders: Bringing Pharma 4.0™ Into the World

Every important cause needs its champion. Champions have a vision of how things should be, and a passion to reach their goals. They are committed and determined to achieve positive results, are willing to do the heavy lifting, and will take consistent and massive action until results are achieved.
The pharma industry certainly has its Pharma 4.0™ champion in Christian Wölbeling, a 56-year-old IT expert. Starting with ideas jotted down on a restaurant napkin, he has tirelessly and expertly led ISPE’s global efforts to define the industry’s slow but sure progression toward a holistic strategy to achieve the Pharma 4.0™ goal.
Born and raised in Hamburg, Germany, Wölbeling took a decidedly unconventional route to his pharmaceutical career. After finishing high school, he enrolled in a technical university where he focused on electronics but soon decided that the course of study was not the right path for him. Feeling that he wanted to “do something practical,” Wölbeling decided to pursue vocational training as a ship’s mechanic and spent two and a half years aboard a vessel. “It was quite inspiring because I learned a lot about technology, and, being confined to a small space for a very long time, I learned about dealing with all kinds of people. On board as crew, we are all one team when it comes to typhoons and hurricanes,” he said.
Following his short career as a seafarer, Wölbeling studied mechanical engineering at the Hamburg University of Applied Sciences, where he completed his master’s degree in 1990. He then started work as a Project Engineer at Blohm & Voss AG, a Hamburg-based shipbuilding and engineering company. After engineering highly efficient power stations that generate both heat and electricity for a year, he realized that he wanted to make a career change when he discovered software was entering the business fields.
One Saturday morning, he opened the newspaper—there were no job websites or LinkedIn in 1992—and came across an advertisement for Werum Software & Systems GmbH, as it was called at that time, based in Lüneburg, Germany. Wölbeling joined the Sales and Marketing team and has since seen Werum grow from an organization of only 65 employees to an international supplier of manufacturing execution systems (MES) software and IT solutions for the pharma and biopharmaceutical industries. When Pharmaceutical Engineering spoke with Wölbeling, he had been at Werum for almost 28 years. He is currently Senior Director of Global Accounts.
Pharma Beginnings
“In the early days, we had some automation software, mainly supervisory control and data acquisition (SCADA) systems, process control, and monitoring and real-time software applications and databases,” recalled Wölbeling. “At a certain point, we received a request for proposal from a pharmaceutical company in eastern Germany (this was shortly after Germany reunified). They were looking for an automation system along with recipe management and material control modules, and we sold them what was at that time known as our PAS-PLS (Process Automation System–Prozess Leit System) and completed the software package by adding weigh and dispense, recipe, warehouse, and quality management modules; that system was the predecessor to our Process Automation System-X product, which today makes us the manufacturing execution systems market leader for pharma and biotech.”
That breakthrough sale was the first of three within Germany and was followed by the company’s first international contract win with Krka, a large international generics manufacturer based in Slovenia. “This was my first experience in an international environment,” said Wölbeling. “I learned a lot about dealing with multinationals with regard to software as well as contract negotiations.” Krka still uses Werum software, he noted.
“Around 2000, we decided we needed to get more global. I had the opportunity to travel to the United States to help set up our first subsidiary there and to get the first orders in. This was my first time selling software in America, and we were fortunate to receive an order from Novartis, which needed a manufacturing execution systems program for a new site in New York state. It became a lighthouse project for us, and it opened the door to all of the big American pharmaceutical companies.”
Becoming an ISPE Trailblazer
Wölbeling first became involved as a member of the ISPE Germany/Austria/Switzerland (D/A/CH) Affiliate in 1998, and a defining moment in his ISPE life came in 2004, when he attended his first ISPE Annual Meeting & Expo in the United States. It was there that he met well-known ISPE member David Selby, who knew that Wölbeling was working in IT and had previously founded an ISPE special interest group (SIG) for process analytical technologies (PAT) within the Germany/Austria/Switzerland Affiliate. At that time, there was a new FDA guideline for process analytical technologies, and Selby asked Wölbeling to join the steering committee for the ISPE process analytical technologies Community of Practice (CoP), as a representative of the local ISPE process analytical technologies activities.
“Of course, I said yes. It was an honor for me; I was still a young guy,” recalled Wölbeling. “From that moment on, I have been involved in process analytical technologies Community of Practice steering committee meetings; later, I also became Co-Chair. Back then, we also had FDA representatives within the Community of Practices, and I got to know former FDA Officer Ali Afnan. It was a really good platform for me to develop my network around automation and process optimization.”
I have educated myself through ISPE, and I have built a huge network, which has been tremendous for me.
Wölbeling noted that the 2004 FDA guideline on process analytical technologies was an early step toward Pharma 4.0™. “When you read through those guidelines, there is al-ready some basic information about the FDA’s expectations regarding data management and data integrity, which is still one of the hottest topics in pharma today.”
Leadership and continuous involvement in committees, both in Europe and globally, have been through lines of Wölbeling’s ISPE membership. He started as a board member of the local Germany/Austria/Switzerland affiliate, and over the years became global steering committee member and chair for several years of the process analytical technologies & Lifecycle Control Strategy Community of Practice, Chair of the ISPE Knowledge Network Council, Co-Chair of the GAMP® manufacturing execution systems special interest group, GAMP® Europe steering committee member at large, and the Founder and Chair of the Pharma 4.0™ special interest group. In addition, he is currently part of ISPE’s European Leadership Team and the Young Professionals Advisor for the Germany/Austria/Switzerland affiliate.
With credentials like these, it’s clear that Wölbeling considers ISPE to be an important aspect of his professional life. “I have educated myself through ISPE, and I have built a huge network, which has been tremendous for me,” he said. “I have known some people I met through ISPE for a very long time, and they have given me a lot of advice. There is always someone I can call to get the information I need. Even if they don’t know, they’ll know someone else who might. ISPE connections have been a big help for me, even in my daily life as I also have many personal friends in ISPE.”
It Started on a Napkin
Great ideas often come to life in unusual ways, and the birth of Pharma 4.0™ was no exception. Sitting in a small restaurant in Basel, Switzerland, in 2015, Wölbeling and fellow ISPE Germany/Austria/Switzerland Board Member Marcel Staudt were discussing fundamental problems of automation, integration, and validation in commercial manufacturing when they started to brainstorm. “It was such a creative moment, and we wrote our ideas on a napkin,” Wölbeling recalled.
The pair discussed the issues inherent in the pharmaceutical industry that set it apart from other industries with a strong manufacturing component, such as the automotive, aviation, and semiconductor sectors. Across industries and around the world, terms like “smart factory,” “factory of the future,” “the industrial internet of things,” and “Industry 4.0” are buzzwords representing a shift in manufacturing concepts driven by digitalization.
Other industries have conquered the challenges of manufacturing high-quality products with a high degree of process automation and real-time quality control by broadly implementing process analytical technologies over many years. “So what is so different in pharma? Are the pharmaceutical regulations really hindering us from process automation and flexible manufacturing?” Wölbeling and Staudt identified one core element of the regulations as key to flexible automated manufacturing: the ICH-defined control strategy (i.e., ICH Q8–Q12).
“From the beginning, Staudt said that the control strategy is what we have to care about, because it defines the product and is executed later in recipes in the manufacturing space,” said Wölbeling. Bridging the automation concepts found in Industry 4.0 with the pharma-specific ICH guidelines would become the root principles behind Pharma 4.0™ to manufacture high-quality products in a flexible and agile way.
As they wrote down their ideas, Wölbeling coined the phrase Pharma 4.0™.
Since that initial dinner, Wölbeling has dedicated countless hours to promoting Pharma 4.0™, giving more than 50 presentations around the globe over the last two years alone, and helping found and run the ISPE Pharma 4.0™ special interest group.
We need to get rid of paper, and we must understand and control the processes based on accurate real-time data. Then we need to break down the silos between product development, manufacturing, engineering, and IT.
The special interest group currently has about 80 active members in working groups, mainly in Europe. “We are getting more involvement and interest from around the globe, and we are currently discussing in ISPE how to organize this best in the interest of the global membership, as a next step,” Wölbeling said.
“When new members join our group, we generally do an onboarding call to explain the root principles,” he explained. “Then they can decide if they want to be part of one of our working groups, which is very valuable because it gives them the opportunity to work on a concrete task on a specific topic.”
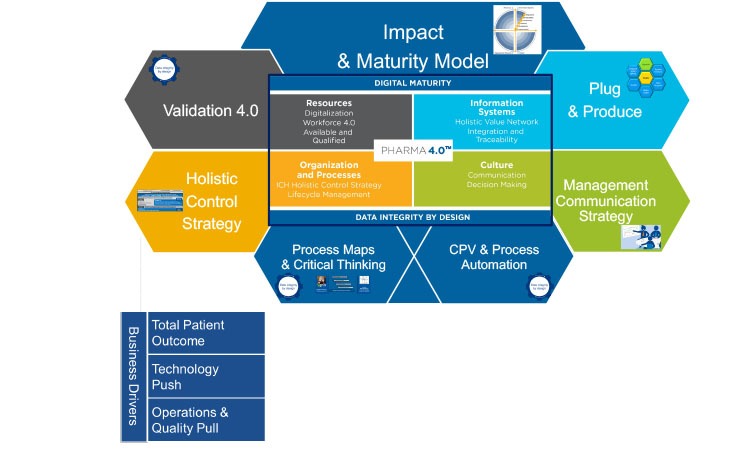
The special interest group has currently six working groups, each corresponding to an element or enabler of the overall Pharma 4.0™ operating model (Figure 1): Holistic Control Strategy from R&D to Commercial Manufacturing; Impact & Maturity Model; Process (Data) Maps & Critical Thinking; Plug & Produce; Management Communication Strategy; and Continued Process Verification (CPV) & Process Automation.
A Lengthy Transition
Wölbeling recognizes that the road to industry-wide Pharma 4.0™ adoption will likely be a long one. “I would say it will take 10 to 15 years,” he said. “I find it fascinating that while the pharma industry is doing very well financially, it is still far behind in automation. The R&D side is more advanced, but when it comes down to what we are producing for our patients, we are far behind. We need to get rid of the paper, and we must understand and control the processes in a flexible and electronic way based on accurate real-time data. A prerequisite for the digitalization for me is to break down the silos between product development, manufacturing, engineering, and IT.”
If we break the silos between quality, operations, and engineering, and allow them to work together, we can make this data-driven holistic control strategy happen; also, we have to digitalize the Pharma Quality System as of ICH Q10. This is what the Pharma 4.0™ operating model is all about.
He also noted that the pharma industry treats quality differently than other industries do. “Quality is always the driver in other industries for efficiency and for delivering a good product. When I look at the car I am driving today, it is completely different from what I drove 10 years ago because the manufacturer has created a new specification for the car for me to buy. When you look at pharma, the product specifications are still the same, and change management for the manufacturing control strategy to improve quality is a lengthy and expensive process. Quality looks at the processes but not creating new processes that produce higher quality and apply new technologies such as process analytical technologies or digitalized processes to the manufacturing control strategy. I think this is a key problem. In consequence, Pharma 4.0™ projects have to be quality- and operations-driven reorganizational projects and not IT projects!”
So, what inspires Wölbeling’s passion for getting industry to adopt Pharma 4.0™? “We have a simple message: There is a way we can do this better,” he said. “Process understanding in the industry is still low, quality by design not very well adopted, and we still have a conservative view on quality. However, if we break the silos between quality, operations, and engineering, and allow them to work together, we can make this data-driven holistic control strategy happen; also, we have to digitalize the Pharma Quality System as of ICH Q10. This is what the Pharma 4.0™ operating model is all about. It is bringing the regulatory piece together with the operations and best practice engineering pieces.”
Globetrotting and Other Adventures
Wölbeling’s career at Werum and his involvement with ISPE have taken him around the globe. He calls himself a “world traveler” and says he and his wife have visited Japan, China, Chile, Canada, and all parts of the United States together. It was not always easy to combine his strong work commitment with his family priorities. Wölbeling has two sons, now in their thirties, and he did not always have enough time for his family. “I am learning to keep a better balance,” he said.
In his spare time, he enjoys running and completed the Munich Marathon four years ago. “I’m not sure I would want to do it again, but it was a very nice experience,” he said. In the winter, he enjoys skiing together with his sons, a sport he has been practicing since he was three years old.
Wölbeling and his wife live in Scharnebeck, a town just northeast of Lüneburg, where he works. They also own a home in Travemünde, which is near the Baltic Sea in the north of Germany. He loves sailing, and at least once a year, he and some friends rent a boat to sail around the Mediterranean or Baltic Sea.
Looking to the Future
Wölbeling sees more promotion of Pharma 4.0TM on the horizon. “So far, we have brought this term to a global level and, together with ISPE, I hope that we can continue to spread it as a good idea,” he said.
An important step in this direction for the Pharma 4.0™ special interest group occurred at the 2019 ISPE Annual Meeting & Expo in Las Vegas, when MHRA representative David Churchward presented on the four MHRA focus topics for the future, including “Digital Health and Pharma 4.0.”
Wölbeling also wants to continue motivating students and Young Professionals to become involved within ISPE. He pointed to the success of Hackathons, which he participated in from the beginning as networking events in Europe, and noted that the first US Hackathon took place at the 2019 ISPE Annual Meeting & Expo. “These are great opportunities for Young Professionals to get in contact with key people in the industry to work on their careers and to improve their skills. I hope to motivate students and Young Professionals to become engaged by volunteering their spare time to an organization like ISPE, because they will see that, in return, they will receive dividends.”
Industry Leader Profiles

Industry Leaders: An Advocate for Quality
Ranjana B. Pathak, BSc (Hons), MBA, DHA, has spent nearly 40 years in the pharmaceutical industry. Currently the President and Global Head of Quality, Medical Affairs, and Pharmacovigilance at Cipla Ltd. in Mumbai, India, Pathak’s long tenure has afforded her an informed perspective on the past, present, and future of the industry.
Industry Leaders: Mission-Driven Leadership
Pam Cheng is Executive Vice President, Global Operations & Information Technology, at AstraZeneca, a United Kingdom–headquartered pharmaceutical company with more than 60,000 employees. In this role, she combines her expertise as an engineer with business savvy and seeks opportunities to lead her company and her industry forward in innovative ways.




