Emerging Leaders has grown from an initiative for interactions among early-career professionals entering ISPE into much more: a training ground, a networking organization, and a new foundation for the future of ISPE and the industry. This article looks at the history of the group, its purpose, current and future initiatives, and a name change that better reflects the path of its members.

Downloads
Emerging Leaders: Nurturing Emerging Talent and the Workforce of the Future
Cover: Emerging Leaders has grown from an initiative for interactions among early-career professionals and entering ISPE into much more: a training ground, a networking organization, and a new foundation for the future of ISPE and the industry. This article looks at the history of the group, its purpose, current and future initiatives, and a name change that better reflects the path of its members.
Toward a Single Global Control Strategy: Industry Study
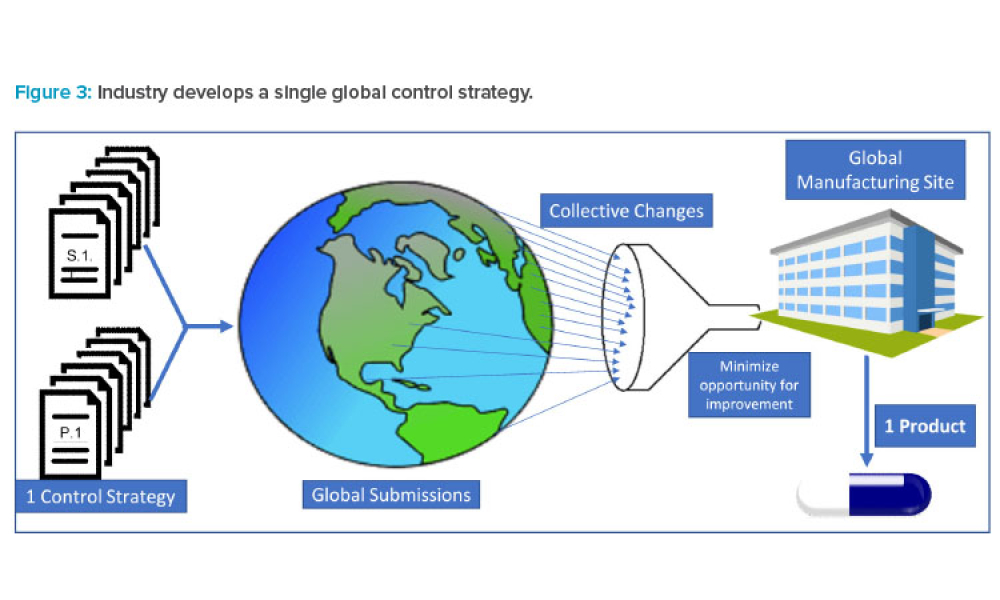
Feature: This article shares data that highlight instances where well-established ICH regulatory members diverge from International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) quality guidance in their evaluation of the same scientific data in chemistry, manufacturing, and controls (CMC) regulatory documents submitted by industry. The data illustrate instances when regulatory divergence led to modifications to control strategies that in turn led to multiple regional and local control strategy variants globally
Measuring Pharma’s Adoption of Industry 4.0
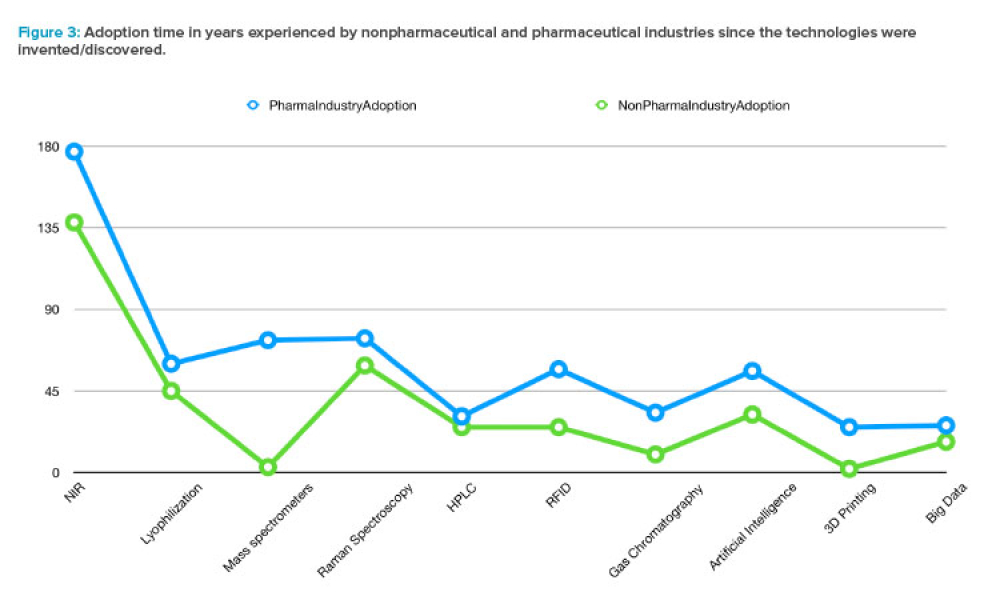
Feature: With the rise of new technologies and predictive analytics capable of handling the huge amounts of data within and across existing information systems, Industry 4.0 has been thriving in many sectors, such as industrial automation, financial technology, retail, and semiconductors. But the health sector in general, and the pharmaceutical industry in particular, has been considered a conservative area, in which innovation has not been adopted as quickly as in other sectors. This article explores how the pharma industry’s adoption of innovation is measured and how the regulated nature of the industry may influence its pace of innovation.
Case Study: Water for Injection Plant AI-based Maintenance
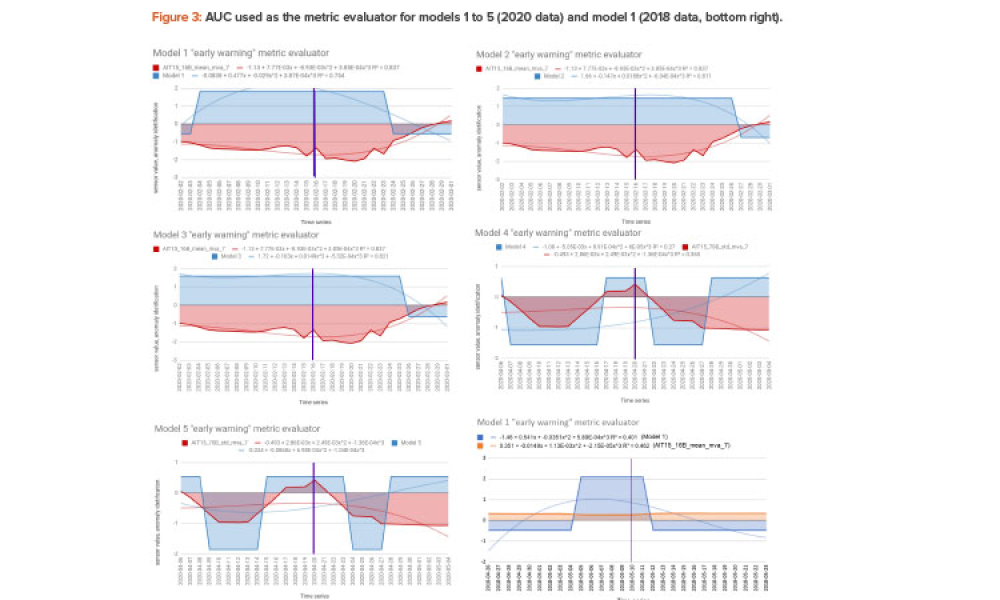
Technical: This article presents the results of applying artificial intelligence (AI) such as machine learning algorithms to identifying and predicting anomalies for corrective maintenance in a water for injection (WFI) processing plant. The aim is to avoid the yearly stoppage of the WFI plant for preventive maintenance activities, common in the industry, and use a more scientific approach for the time between stoppages, expected to be longer after the study and thus saving money and increasing productivity.
In This Issue
Who would have thought at the beginning of the pandemic that we would have several highly effective vaccines against the coronavirus and billions of doses administered by the end of 2021? I am still amazed what we as the pharmaceutical industry have achieved.
ISPE Women in Pharma® (WIP) wants to kick off the new year by focusing on your journey in taking care of your total self. Whether it is your professional or personal mission to better yourself, Women in Pharma®...
The past two years have turned things upside down and forced change in a way that has made many aspects of our lives become unfamiliar. The unforeseen stresses of these changes have pushed many people and companies to their breaking points. Some of these challenges were expected, but came on like a train that we couldn’t stop. I’ve been there, feeling like I don’t have the tools to deal with...
The traditional passing of the gavel took place in person at the 2021 ISPE Annual Meeting & Expo on 2 November. The
ISPE’s Carolina-South Atlantic (CaSA) Chapter serves members from six different states in the US: North and South Carolina, Tennessee, Alabama, Georgia, and Florida, each with their own unique contributions to the pharmaceutical industry.
Since the first edition of the ISPE Good Practice Guide: Good Engineering Practice (GEP) was published in 2008, the pharmaceutical industry and regulators developed industry standards, best practices, and regulatory guidance around topics that relate to Good Engineering Practice, culminating in particular in the ISPE Baseline Guide: Commissioning & Qualification (Second Edition). The...
This article presents the results of applying artificial intelligence (AI), such as machine learning algorithms, to identifying and predicting anomalies for corrective maintenance in a water for injection (WFI) processing plant. The aim is to avoid the yearly stoppage of the water for injection plant for preventive maintenance activities, common in the industry, and use a more scientific...
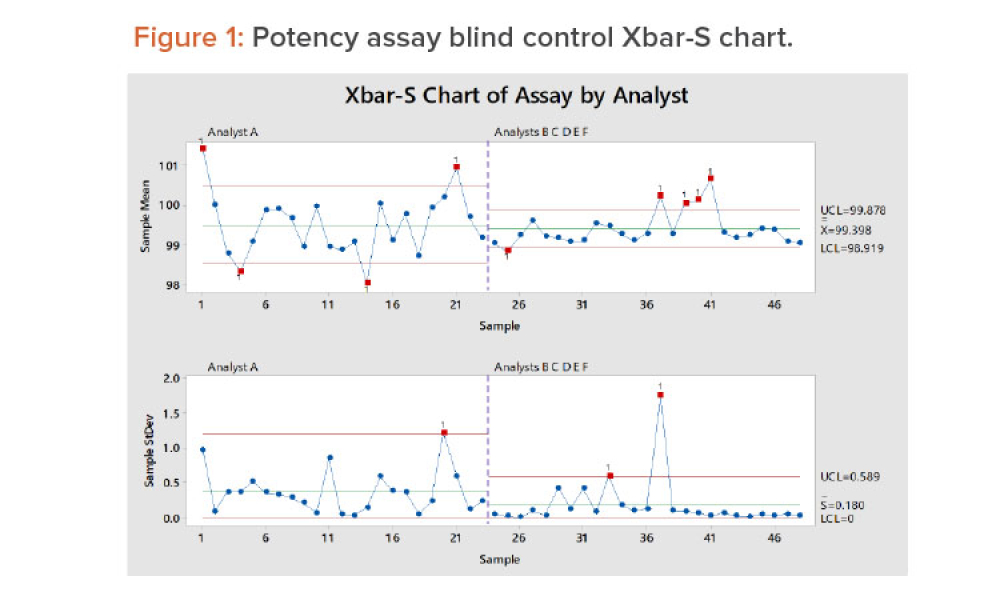
Good data are a characteristic of good science. Quality data are arguably more important today than ever before and are considered by many to be a corporate asset because they are used to develop products and processes, control our manufacturing processes, and improve products and processes when needed.1
During the past decade, industry has experienced a proliferation of regulatory divergence regarding the interpretation and implementation of International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines (and control strategies) across geographic regions. This article shares data that highlight instances where well-established ICH...
With the rise of new technologies and predictive analytics capable of handling the huge amounts of data within and across existing information systems, Industry 4.0 has been thriving in many sectors, such as industrial automation, financial technology, retail, and semiconductors. But the health sector in general,1
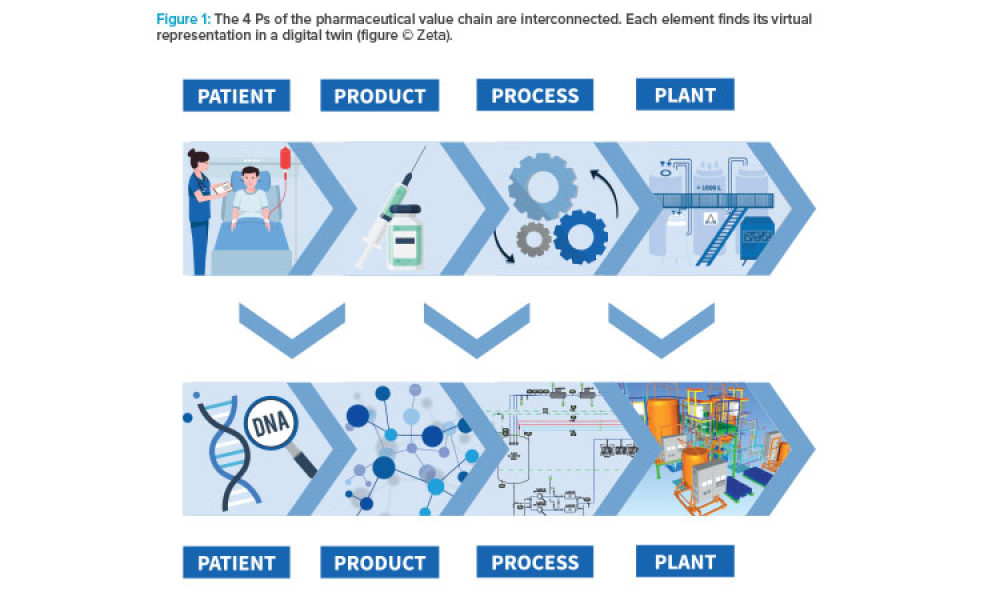
Developing comprehensive digital solutions is crucial for the entire value creation process for pharmaceuticals. A holistic view of the interrelations of product, production process, and plant is becoming increasingly significant. In this context, the application of model-based technologies provides support in drug development, process scale-up, and manufacturing. Furthermore, it accelerates...