It is a great honour and pleasure for me as the 2022-2023 ISPE Board Past Chair and Chair of the 2023 ISPE Aseptic Conference Program Committee to give you a first glimpse at the program and of the information and...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
ISPE’s Transportable/Point of Care (PoC) Manufacturing Technology team, a working group under the Product Quality Lifecycle Implementation (PQLI)® committee, is presenting a session entitled Transportable, Small Footprint Biopharmaceutical Manufacturing Facilities: A Regulatory Perspective at the 2022 ISPE Annual Meeting & Expo on Monday, 31 October at 1100–1230 as part of the...
Helping people, projects and companies succeed are the aspects Dharti Pancholi has enjoyed most about her career in the pharmaceutical industry. “I really like empowering people whether it is my team or my clients. It is great to bring together people who have a diverse set of experiences, skills, or perspectives, who might not normally work together, and collectively develop something, grow a...
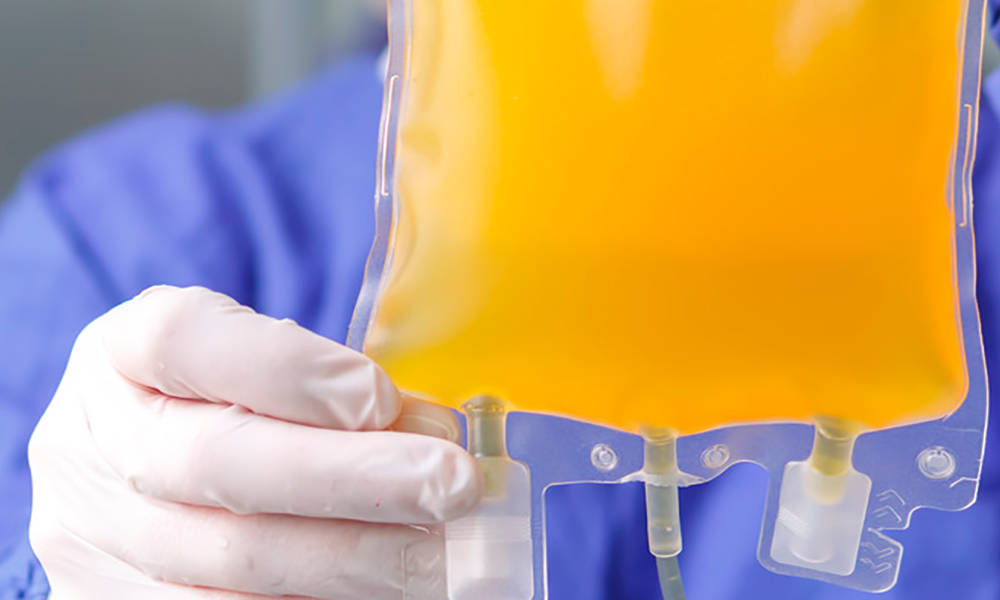
Global demand for plasma-derived therapies (PDTs) continues to swell—the compacting result of an aging population, clinical successes, and recent applications for both widespread diseases and multiple rare conditions. Serving these needs has been a significant challenge over the last two and a half years, with a decline in plasma donations due to the COVID-19 pandemic. The donation shortage...
Honorable Mention recognizes projects that did not win a specific category but were clearly successful projects that overcame significant challenges in planning, execution, and delivery.
Emerging Leaders (EL) within the pharmaceutical industry have been invited from around the world to participate in the 2022 ISPE Annual Meeting & Expo Program Committee. This unique opportunity allows...
On 16 June 2022, the Project Management Community of Practice Benelux (PM CoP Benelux) hosted the “Need for Speed in Pharma,” event. There were more than 70 attendees at the event from Belgium and the Netherlands which was held at the MSD Animal Health facility in Boxmeer, Netherlands.
The energy was felt within the room as more than 30 engaged women and allies came together in support of ISPE D/A/CH affiliate’s launch of Women in Pharma® on 8 September 2022 at the Bayer Consumer care’s headquarters in Basel, Switzerland. The event consisted of an informational session, lunch, and moderated workshop to give...
Winners in this category exemplify application of novel approaches, standards, and practices which result in efficient processing, resourceful utilities, and business advantage by increasing patient access and preventing drug shortages through in-country-for country manufacturing; outbreak, epidemic, or emerging health crisis response via rapid deployment and fast-track drug production; and...
Annex 1 of the EC GMP Guide "Manufacture of Sterile Medicinal Products" has a long history. First published in 1989, there have been a total of 5 adaptations in 1996, 2003, 2005, 2007 and 2009, but no complete revision. In 2012, there was a proposal for a complete revision which resulted in a concept paper in 2015. The first draft for comments from industry stakeholders was...
It may seem that the only things loved by pharma industry more than acronyms are new buzz phrases. And that may be true. But sometimes, behind the buzz phrase, there are real, strong, achievable benefits to our work, our industry, and our end patients.
Single-use plastics are critical for healthcare, life sciences laboratories, and bioprocessing. They have many benefits over other materials and their use is increasing. In particular, single-use technologies for bioprocessing decrease costs, reduce water and energy usage, and increase efficiency and scalability. However, most of these high-value, virgin materials end up in landfills and...
The industry is continuously evolving to meet new requirements. Many of these requirements include adhering to global regulatory harmonization, improving supply chain robustness, minimizing drug shortages, reducing the complexity of managing product life cycles, and reducing the environmental impact of pharmaceutical processes. At the
Winners in this category exemplify the novel application of process manufacturing techniques, innovative design concepts, new technologies and unique solutions that exemplify the next generation of agile, flexible, efficient and effective new and existing pharmaceutical and biotechnology facilities. This includes implementation of commercially available and custom developed equipment which...
Winners in this category exemplify application of novel approaches, standards, and practices which result in efficient processing, resourceful utilities, and business advantage by increasing patient access and preventing drug shortages through in-country-for country manufacturing; outbreak, epidemic, or emerging health crisis response via rapid deployment and fast-track drug production; and...
Do you have your finger on the pulse of regulatory concerns in the Asia-Pacific Region? Would you like to discuss strategies to meet regional challenges? Are you interested in regulatory harmonization? If so, we’d like to re-introduce ISPE’s Regulatory Quality Harmonization Committee (RQHC)’s Asia-Pacific Regional Focus Group (A-P RFG).
At the 2022 ISPE Europe Annual Meeting held in Madrid on 25-27 April 2022, Evdokia Korakianiti, Head of Quality and Safety, European Medicines Agency (EMA), gave a keynote presentation of EMA’s approach to...
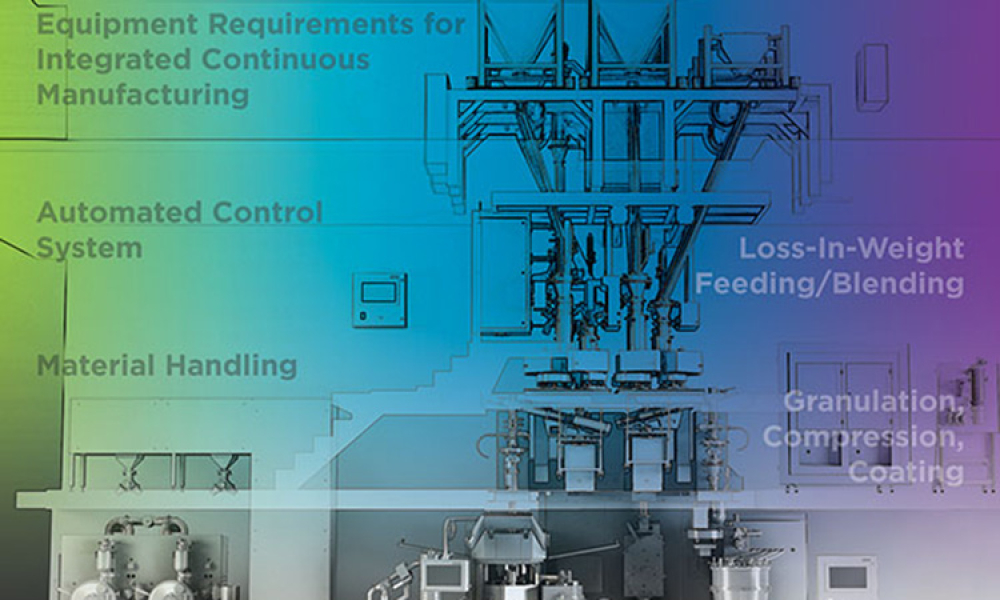
As the benefits of continuous manufacturing became more apparent in the pharmaceutical industry, companies looked for ways to incorporate the technology into their manufacturing processes. In 2017 The ISPE Oral Solid Dosage (OSD) Community of Practice (CoP) formed a working team to advance the use of continuous manufacturing in the pharmaceutical industry and to increase the long-term...