The 2022 ISPE Pharma 4.0™ Emerging Leader Hackathon event was organized by the ISPE Emerging Leaders DACH Affiliate in collaboration with the ISPE Pharma 4.0™ Community of Practice (CoP) Plug and Produce Subcommittee. The goal was to bring together students and young professionals from different fields of study and countries together to explore the emerging challenges and opportunities in...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
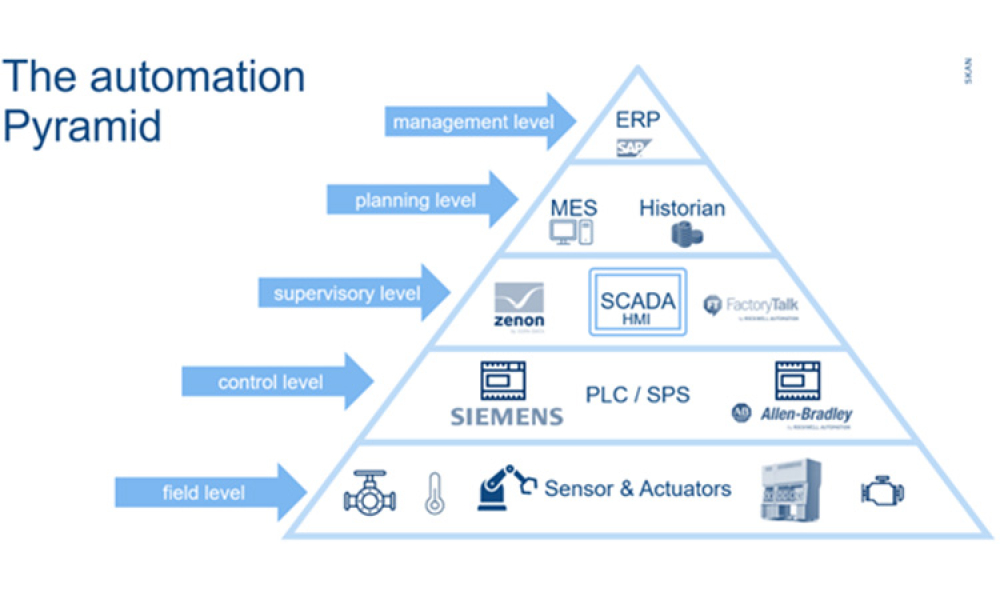
The lifecycle of interfaces is of crucial importance for the interoperability of systems from different technical and organizational areas within or between companies.
Interfaces are the integration points of connected systems for the transmission of master or transaction data including status updates. Interfaces are typically parts of data streams or data hierarchies, e.g., Lab...
The pharmaceutical industry is rapidly evolving. The major trends in the recent years are (1) fully automated production such as continuous manufacturing and (2) the adoption of digitalized processes for paperless production. Both approaches have significant implications for Annex 1 compliance and the role of Contract Manufacturing Organizations (CMOs) in production facilitating these...
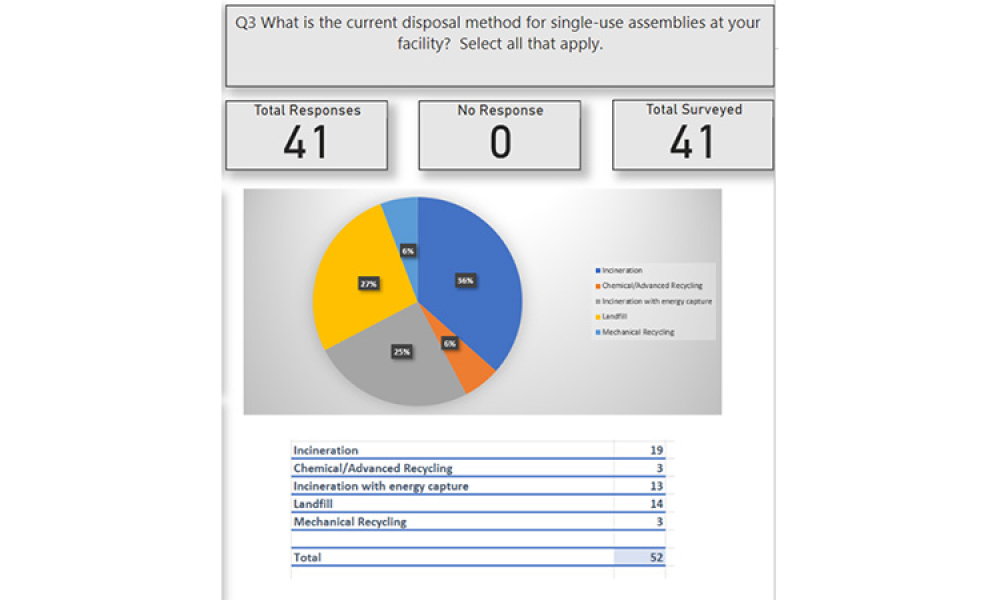
Users Perspective on End of Life Management for Single-Use Products used in Bioproduction
ISPE invites Emerging Leaders and Students from all over the globe to join us at this unique virtual event to explore the ISPE Communities of Practice (CoPs) and meet with the leaders of the CoP Steering Committees. This provides an opportunity to engage with industry leaders, learn about the various CoPs, and find out how you as an Emerging Leader or Student can get more involved.
Arriving in the United States at the age of 17 to pursue my dreams was one of the greatest challenges of my life. It was through this experience that I learned the importance of challenging my perspective. This was made possible through my involvement with ISPE, and four years later, I’m proud to announce the launch of Mentor ISPE.
Pharmaceutical and biotech portfolios continue to transition from high volume blockbusters to a diversified set of lower volume, targeted therapeutics. At the same time, many companies are pursuing localized manufacturing to adjust to supply chain challenges and to improve access to medicine. The industry must also address increased pricing pressure due to inflation and government policies....
GAMP® 5 (Second Edition) was published on 29th July 2022 and was presented and discussed at the 2023 ISPE Annual Meeting and at several local...
Here’s How Our Members Intend on Making It a Reality…
Each International Women’s Day, the world comes together to celebrate the social, economic, cultural, and political achievements of women, calling for accelerated equality across borders. International Women’s Day has taken place for over a century, with the first taking place in 1911 and having the support of over a million...
Just off the heels of ISPE’s Facilities of the Future Conference, which took place this past February, ISPE’s Women in Pharma continues to leverage its momentum to amplify the group’s mission to create a more equitable pharmaceutical industry on a grand scale.
The second day of the 2023 ISPE Facilities of the Future Conference in North Bethesda, Maryland, included keynotes addressing various facets of the industry and their impact on future facilities planning and development. Key among these included a presentation by the director of FDA’s CBER on cell and gene therapy manufacturing and a range of considerations behind new facility planning from a...
The updated EU GMP Annex 1 Revision: Manufacture of Sterile Medicinal Products released in August 2022 introduced changes which require more stringent sterile manufacturing processes, including implementing a contamination control strategy and new technology to reduce the risk of contamination. Regulators discussed those changes and how they impact manufacturing practices during panel...
ISPE is launching a new program, Enabling Global Pharma Innovation: Delivering for Patients in support of many regulatory agencies’ ambitions to promote introduction of innovative pharmaceutical manufacturing. It is incumbent for industry to modernize and innovate pharmaceutical manufacturing to improve efficiency and increase confidence in quality assurance for the benefit of...
What is Commenting?
As regulatory agencies invite public comment on a new or revised regulation or guidance, they look to ISPE for input on the latest scientific and technical developments. ISPE submits official comments when we are able to provide that input.
ISPE’s commenting process is overseen by ISPE’s Regulatory Quality Harmonization Committee (RQHC)...
With the widespread use of paperless validation software application comes the need to present digital CQV content to inspection professionals. This blogpost will summarize inspection topics for presenting digital CQV content and provide a list of common questions encountered during inspection events.
ISPE’s long-time Asia-Pacific Regulatory Advisor Bob Tribe retired from ISPE at the end of 2022, bringing to a close more than 18 years of service to the Society.
ATMPs, Cell and Genetic therapies - or whatever other jurisdictions use – have come a long way. I am unsure how many of us have forgotten the early failures and setbacks. There was a time, in the early years, when serious side effects, such as deaths, endangered the field. It prompted the regulators to halt research, introducing various constraints and safeguards. In Europe, it felt like every...