This article focuses on pragmatic quality- and risk-based approaches to IT infrastructure. It covers recommendations made by a US FDA/industry team linked to the US FDA Center for Devices and Radiological Health (CDRH) Case for Quality initiative1

Downloads
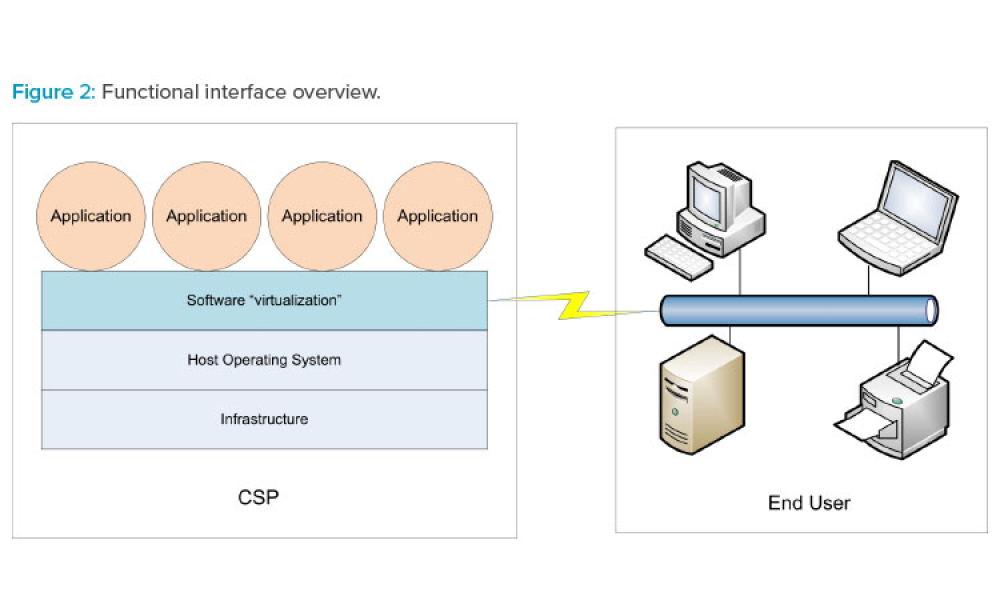
IT Services: Applying Good IT Practice and Automation
Cover: This article focuses on pragmatic quality- and risk-based approaches to IT infrastructure. It covers recommendations made by a US FDA/industry team linked to the US FDA Center for Devices and Radiological Health (CDRH) Case for Quality initiative—which is promoting a risk-based, product quality, and patient-centric approach to computerized systems assurance—as well as the GAMP® reexamination of approaches to IT infrastructure control and compliance.
Best Practices for Deploying Real-World Evidence Solutions
Feature: Real-world evidence (RWE) is clinical evidence regarding the usage and potential bene ts or risks of a medical product derived from analysis of real-world data (RWD) relating to patient health status and the healthcare delivery. RWE helps healthcare companies better understand and establish stronger evidence of products’ performance, clinical value, and cost-effectiveness outside the controlled environment of clinical trials. Outcome-based studies are increasingly depending on RWD and RWE to speed up drug development and approvals, and ultimately reduce development costs.
Happy 30th Anniversary to the GAMP® Community of Practice!
Feature: In 2021, the ISPE GAMP® Community of Practice (CoP) is celebrating 30 years of promoting industry good practice for computerized systems and encouraging technical innovation and progress, while protecting patient safety, product quality, and data integrity.
Analyzing System Performance through Process Instrumentation Data
Technical: What if the reliability of a system could be improved by accessing the standard data provided with modern process instrumentation? These data, accessed from existing instrumentation, can be used to analyze the fitness of processes, equipment, and instruments; better understand processes; support discrepancy investigations; and provide a data-driven basis for the timing of maintenance and calibration. This article covers a few particularly illustrative examples in detail.
In This Issue
I used to think “home detention” was no punishment at all, but this last year has dramatically changed my thinking. Despite working from home for over a year, my house is a wreck! Like many of you, I feel busier than ever, despite not traveling and not driving to and from work. Disruption used to be rare and now it is common: having to adapt to new ways of doing most everything, like buying...
This year’s Women in Pharma® (WIP) theme of “Fueling the Fire” harnesses the energy and passion that drive women to achieve career advancement, personal growth, and satisfaction. The 3 March webinar focused on the Diversity...
2020 was a year for finding new ways of working for the entire ISPE Emerging Leaders (EL) community. In February, Emerging Leaders stepped up the challenge by holding the first fully
Real-world evidence (RWE) is clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data (RWD) relating to patient health status and the healthcare delivery.1
In 2021, the ISPE GAMP® Community of Practice (CoP) is celebrating 30 years of promoting industry good practice for computerized systems and encouraging technical innovation and progress, while protecting patient...
Cloud computing can be described as networked access and utilization of configurable computing resources such as data and information storage, processing capabilities, applications, and other services on computerized systems provided and/or maintained by a remote organization. As life sciences companies consider the advantages and costs of utilizing cloud services, they first need to invest...
This article provides a beginner’s overview of how organizations can achieve a state of preparedness (readiness) for inspections, with a specific focus on IT systems.
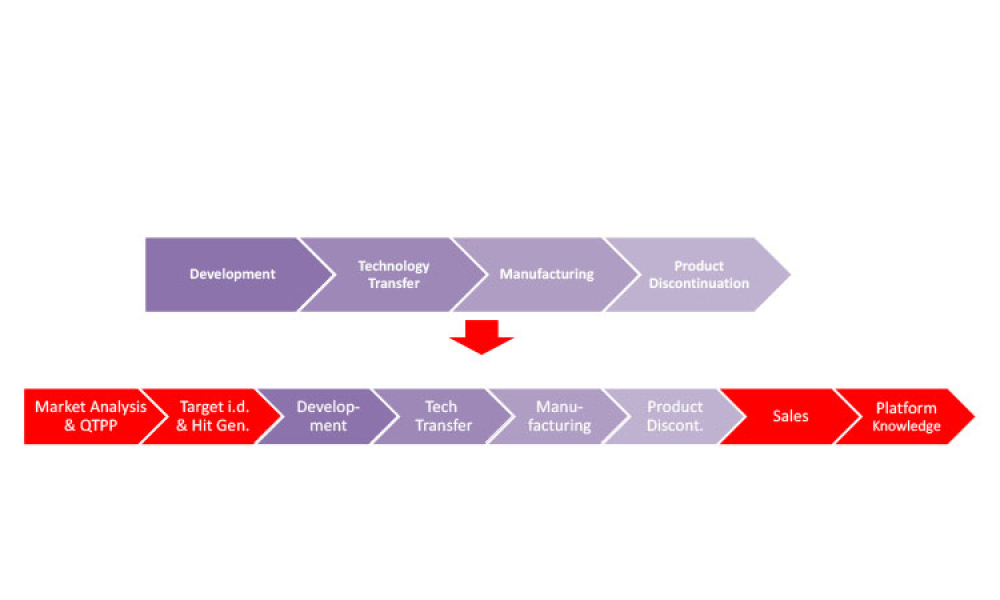
This second of a two-part series explores digital transformation and digitalization in the biopharmaceutical industry with information about how data science enables digitalization along the product life cycle. (Part 1 was published in the March-April 2021 issue of Pharmaceutical Engineering.1
ISPE Emerging Leaders (ELs) held the first fully virtual International Hackathon in February. Fifty-one participants from over 22 countries encountered real-life challenges with working remotely and across time zones. Innovation...
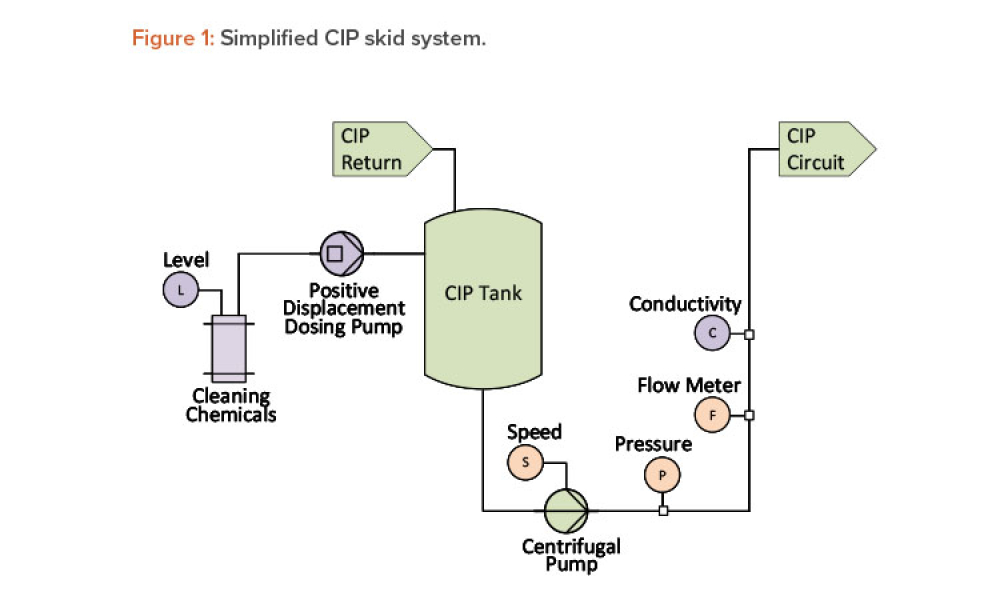
Maintenance can impact both the quality of products and the compliance of pharmaceutical processes, and maintenance programs have long been recognized as critical to the success of the operations they support.
What if the reliability of a system could be improved by accessing the standard data provided with modern process instrumentation? These data, accessed from existing instrumentation, can be used to analyze the fitness of processes, equipment, and instruments; better understand processes; support discrepancy investigations; and provide a data-driven basis for the timing of maintenance and...
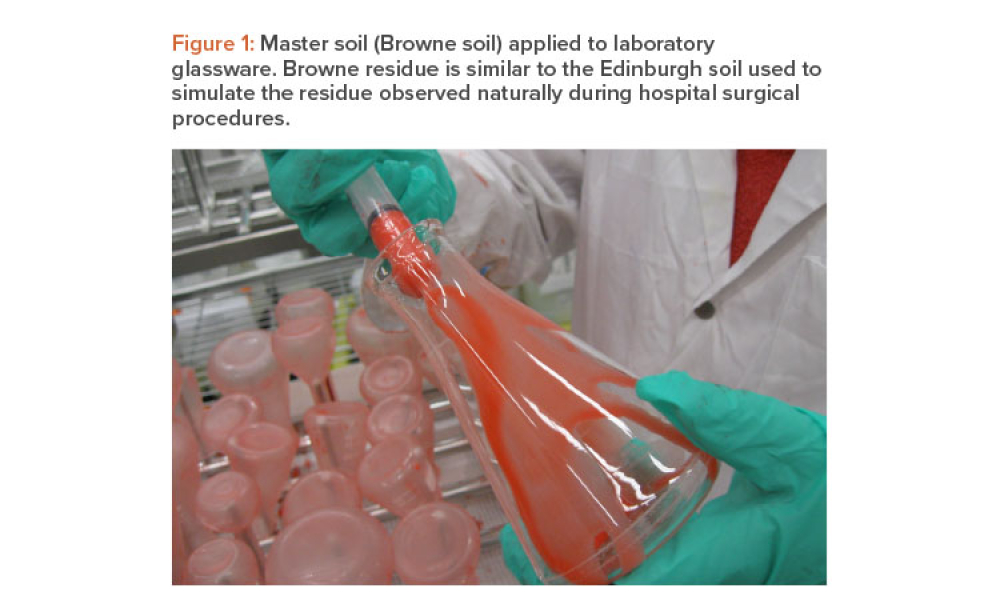
One of the goals of the cleaning validation design phase is to define critical process parameters (inputs) and acceptance criteria (outputs) of the cleaning process. This article explores the selection of a master soil as part of the cleaning validation design phase for automated parts washers. The selection and qualification of a master soil through laboratory testing and during factory...
The 30th ISPE Aseptic Conference culminated in the interactive regulatory panel, which offered attendees the unique opportunity to ask questions directly to the regulators.