Commissioning and Qualification (C&Q) are terms and processes related to the manufacturing of pharmaceutical or biotechnology products. Each term represents a scope of work that is part of a larger framework for making sure that a facility —and the equipment in it— will function as required and be approved by the regulatory agencies that have jurisdiction over that facility. Produced by pharmaceutical manufacturing industry professionals, ISPE offers a variety of resources to help narrow interpretation of regulatory standards for improved compliance and quality, efficiency, and cost reductions.
Guidance Documents
Commissioning & Qualification (5)
- Good Practice Guide: C&Q of Pharma Water & Steam Systems 2nd Edition
- Good Practice Guide: Decommissioning Pharma Equipment & Facilities
- Good Practice Guide: Controlled Temperature Chambers 2nd Edition
- Baseline Guide Vol 5: Commissioning & Qualification 2nd Edition
- GAMP Good Practice Guide: Testing GxP Systems 2nd Edition
Critical Utilities (1)
Data Integrity (1)
Quality by Design (1)
Sustainable Facilities, HVAC, & Controlled Environments (1)
Community Discussions
Community Discussions
Feb 16, 2025
Workforce of the Future
Feb 15, 2025
Manufacturing Operations
Feb 13, 2025
Manufacturing Operations
Oral Solid Dosage
Feb 10, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Feb 06, 2025
Feb 03, 2025
Jan 30, 2025
Webinars
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
Commissioning and Qualification Training Course
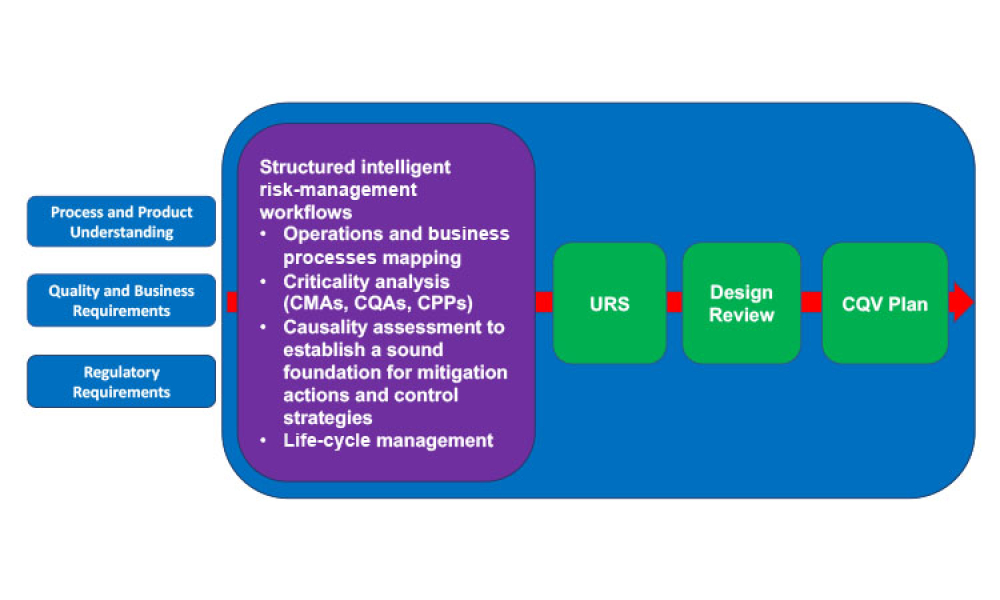
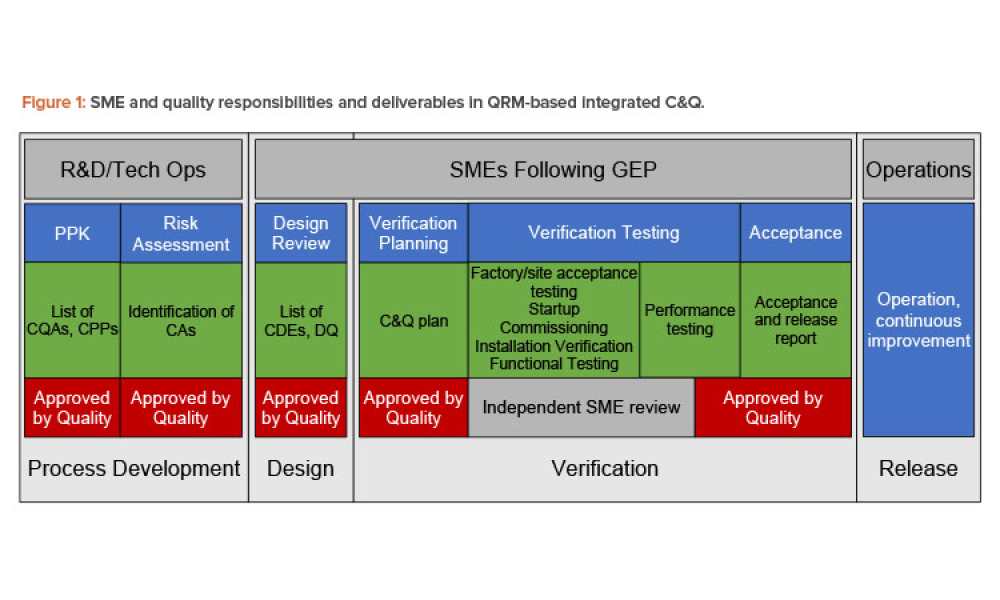
Worldwide Regulatory expectations and guidance as led by FDA and the EU have stated that all Pharmaceutical Quality Systems should apply a QRM (Quality Risk Management) approach. Through interactive workshops, this course will explain and apply the science and risk-based approach to integrated lifecycle Commissioning & Qualification by conducting verification of systems, equipment and facilities in accordance with the recently issued 2nd Edition Guide, ICH documents Q8 (R2), Q9, and Q10, current Regulatory Guidance, industry best practices, and ASTM E2500.
Facilities Management Training Course
This interactive course will provide the learner with tools on how to implement a sustainable approach to a risk-based C&Q program, integrate the new C&Q program into existing quality systems, Quality Assurance and Engineering Management Systems and define organizational capabilities to support the new C&Q program.
Pharma Facilities Project Management Training Course
Specifically targeted to the needs of facility projects within the regulated pharmaceutical industry and demonstrates the value inherent in the use of “good practice” project management in the regulated pharmaceutical environment.
Pharmaceutical Job Board
Featured Conferences