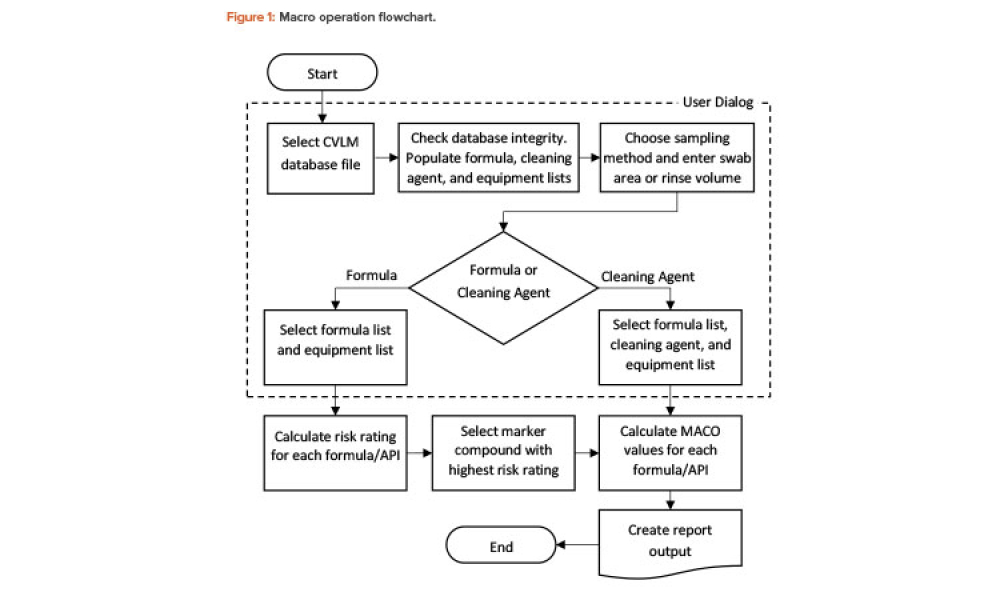
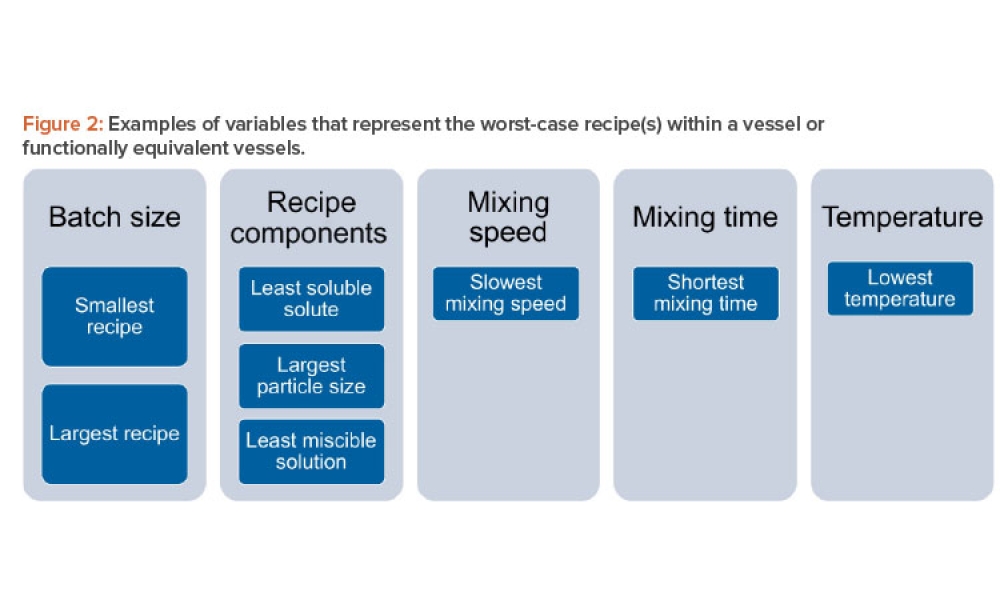
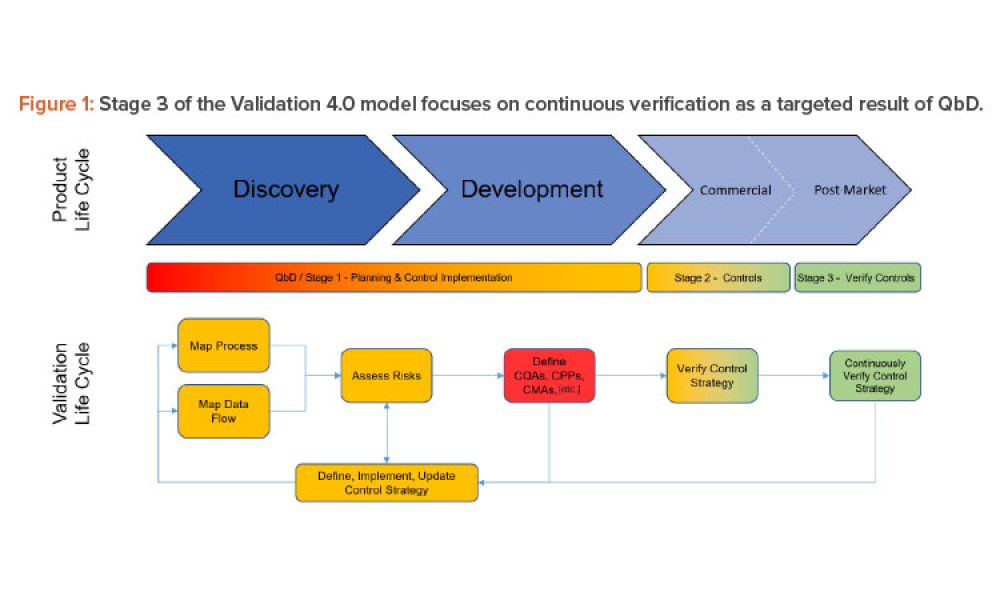
Validation is creating an evidence trail to show that an action, method, or system leads to a consistent and reproducible result. Validation is the collection and evaluation of data from the process design stage through commercial production, which establishes scientific evidence that a process or components of a process can consistently deliver a quality product. Process validation involves a series of activities taking place over the lifecycle of the product and process.
Guidance Documents
Data Integrity (11)
+GAMP® (12)
+Lifecycle Management (1)
+Microbiological & Viral Contamination Control (1)
+Quality Assurance (1)
+Validation (14)
+Community Discussions
Community Discussions
Apr 08, 2025
Validation
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Feb 03, 2025
Jan 27, 2025
Dec 04, 2024
GAMP®
Lifecycle Management
Oct 31, 2024
Regulatory
Quality
Good Manufacturing Practice
Sustainable Facilities, HVAC, & Controlled Environments
Oct 30, 2024
Validation
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
White Papers
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…
Lifecycle Approach to Biotech Process Validation
This discussion paper proposes ideas for answering the questions about the application of the…
Stage 3 Process Validation: Applying Continued Process Verification Expectations
This discussion paper proposes ideas for answering the questions “How is Stage 3 monitoring and…

Pharmaceutical Job Board
Videos
iSpeak Blog Posts
Featured Conferences