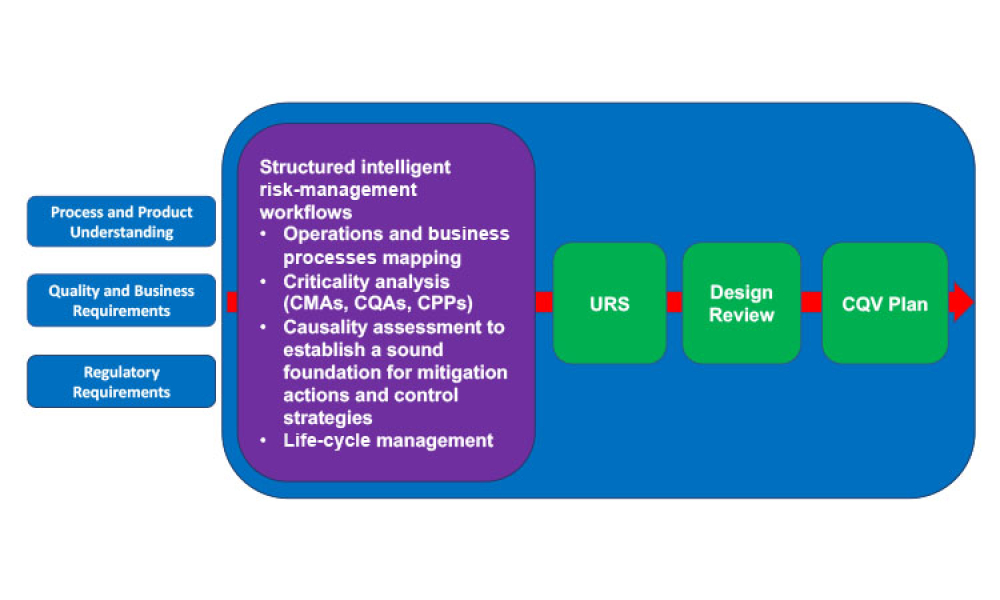
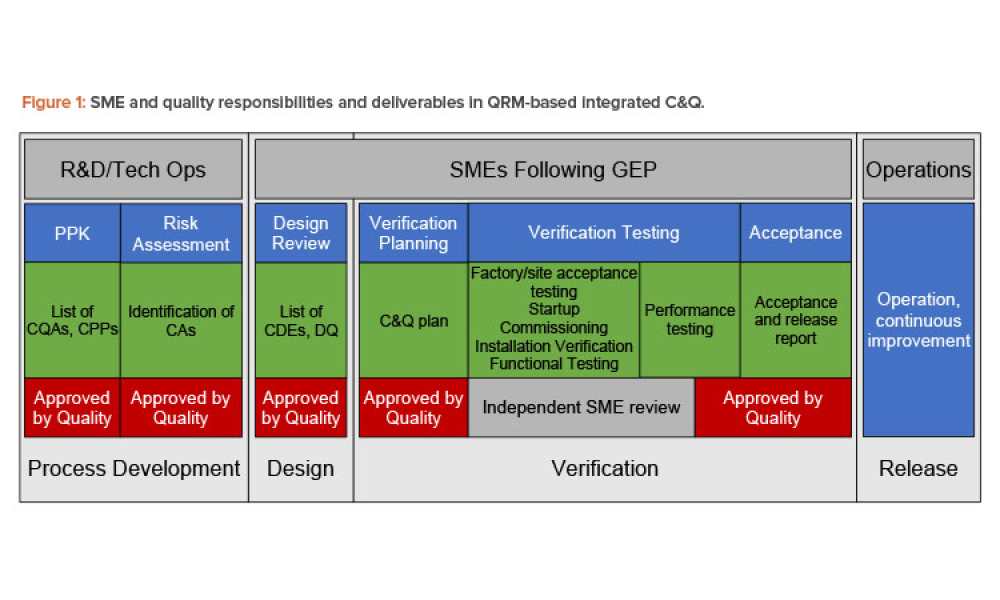
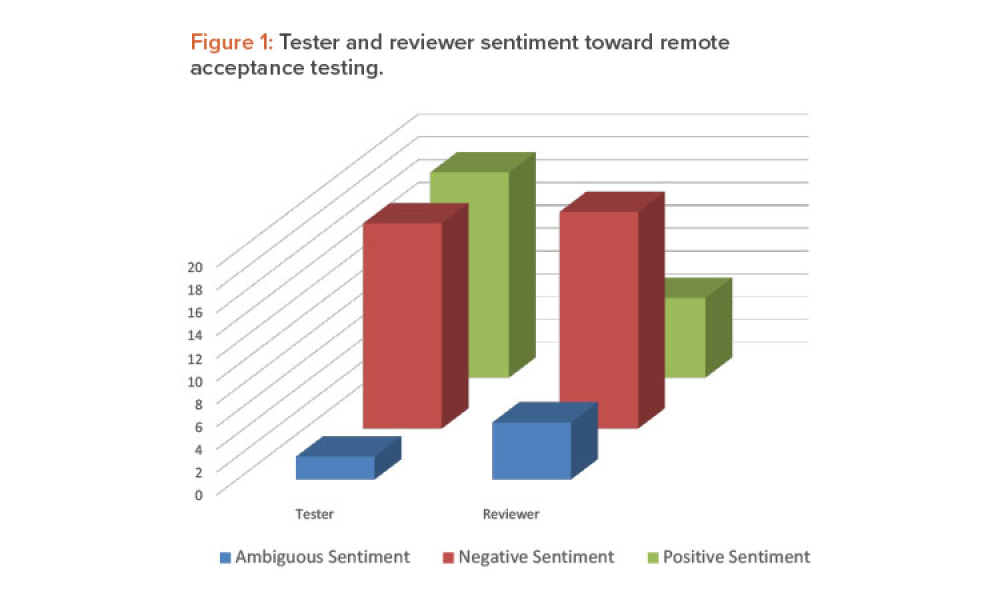
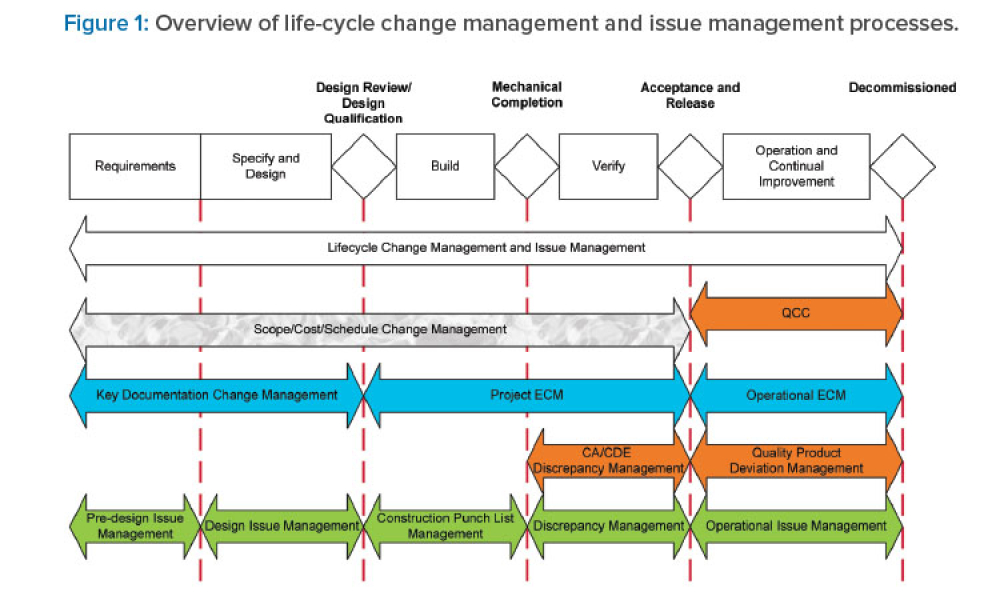
Commissioning and Qualification (C&Q) are terms and processes related to the manufacturing of pharmaceutical or biotechnology products. Each term represents a scope of work that is part of a larger framework for making sure that a facility —and the equipment in it— will function as required and be approved by the regulatory agencies that have jurisdiction over that facility. Produced by pharmaceutical manufacturing industry professionals, ISPE offers a variety of resources to help narrow interpretation of regulatory standards for improved compliance and quality, efficiency, and cost reductions.
Guidance Documents
Commissioning & Qualification (5)
+Critical Utilities (1)
+Data Integrity (1)
+GAMP® (1)
+Quality by Design (1)
+Regulatory (1)
+Sustainable Facilities, HVAC, & Controlled Environments (1)
+Community Discussions
Community Discussions
Apr 08, 2025
Validation
Apr 08, 2025
Data Integrity
Apr 07, 2025
Advanced Manufacturing
Biotechnology
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Mar 20, 2025
Mar 20, 2025
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
Commissioning and Qualification Training Course
+Facilities Management Training Course
+Pharma Facilities Project Management Training Course
+Pharmaceutical Job Board
Featured Conferences