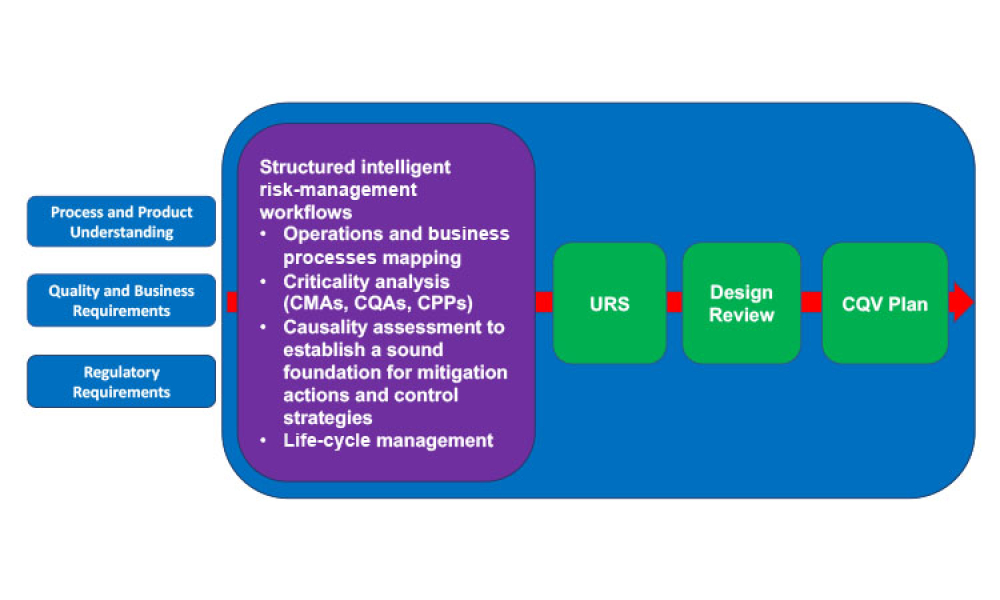
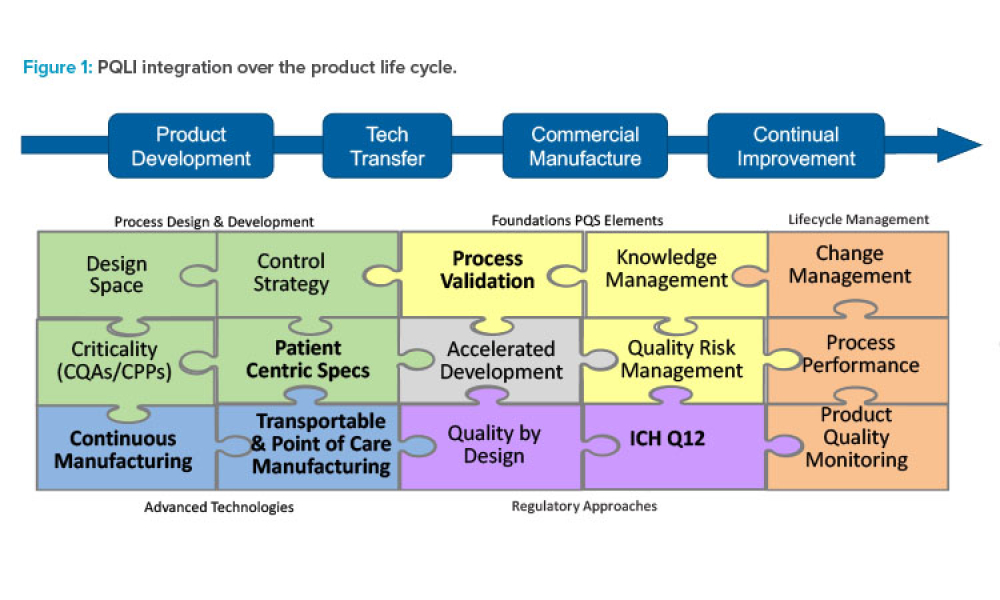
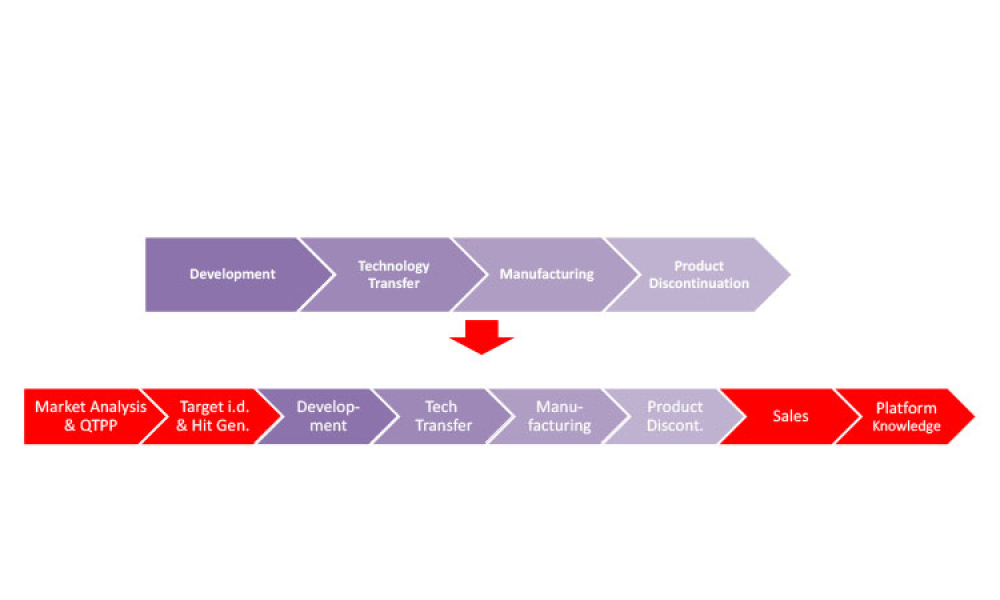
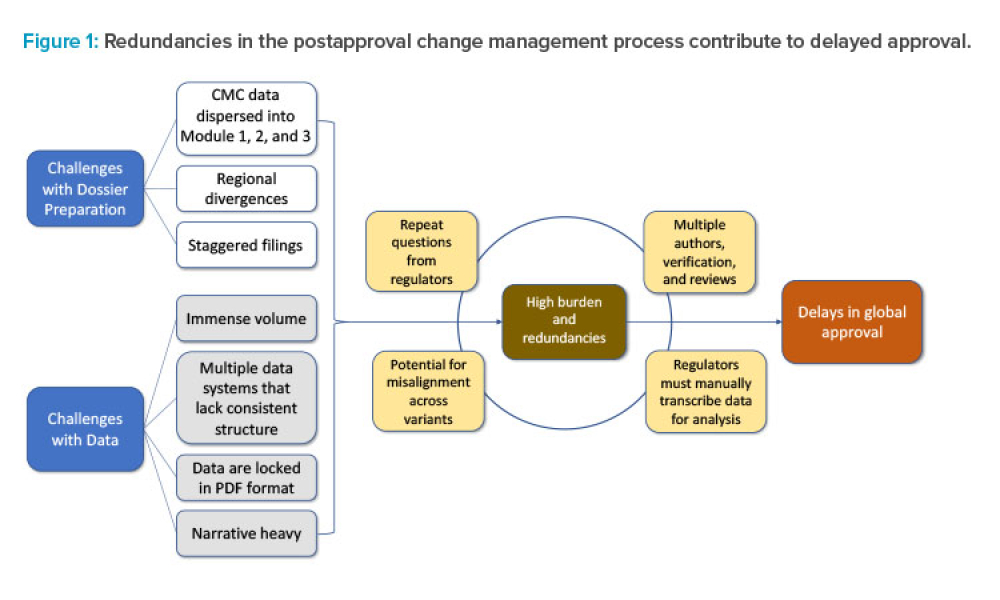
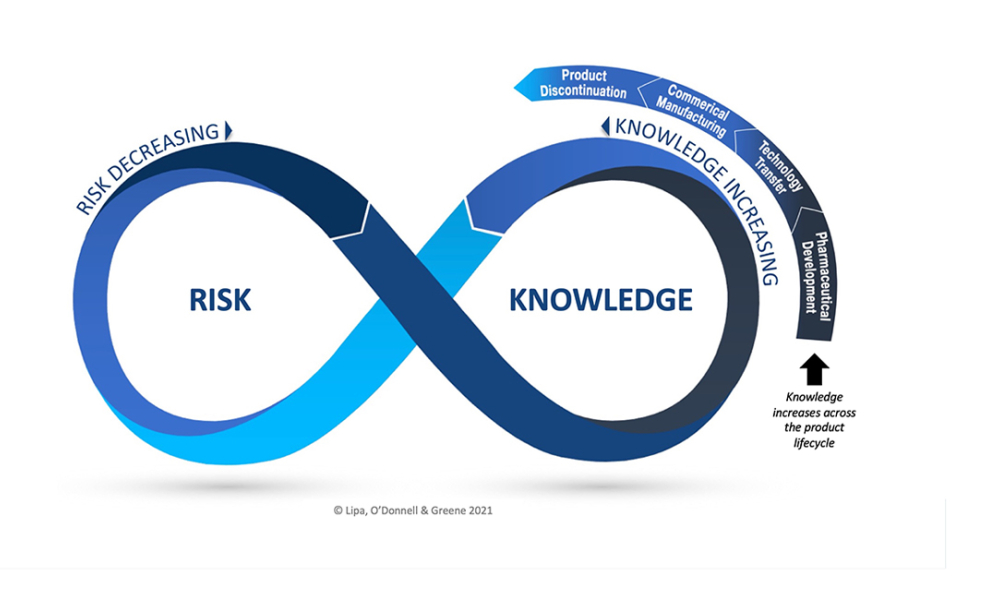
Pharmaceutical Product Lifecycle Management is the process of managing the entire lifecycle of a product from its conception, through design and manufacture, to service and disposal. Lifecycle Management forms the product information backbone for a company and its extended enterprise.
Guidance Documents
Advanced Manufacturing (1)
+Biotechnology (2)
+GAMP® (1)
+Knowledge Management (1)
+Lifecycle Management (6)
+Manufacturing Operations (1)
+Process Analytical Technology (3)
+Quality by Design (2)
+Validation (1)
+Community Discussions
Community Discussions
Apr 24, 2025
Validation
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Apr 08, 2025
Data Integrity
Apr 07, 2025
Advanced Manufacturing
Artificial Intelligence
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Artificial Intelligence
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
White Papers
January / February 2025
GAMP® is indispensable for safeguarding the safety, quality, and compliance of pharmaceutical…
Stage 3 Process Validation: Applying Continued Process Verification Expectations
This discussion paper proposes ideas for answering the questions “How is Stage 3 monitoring and…