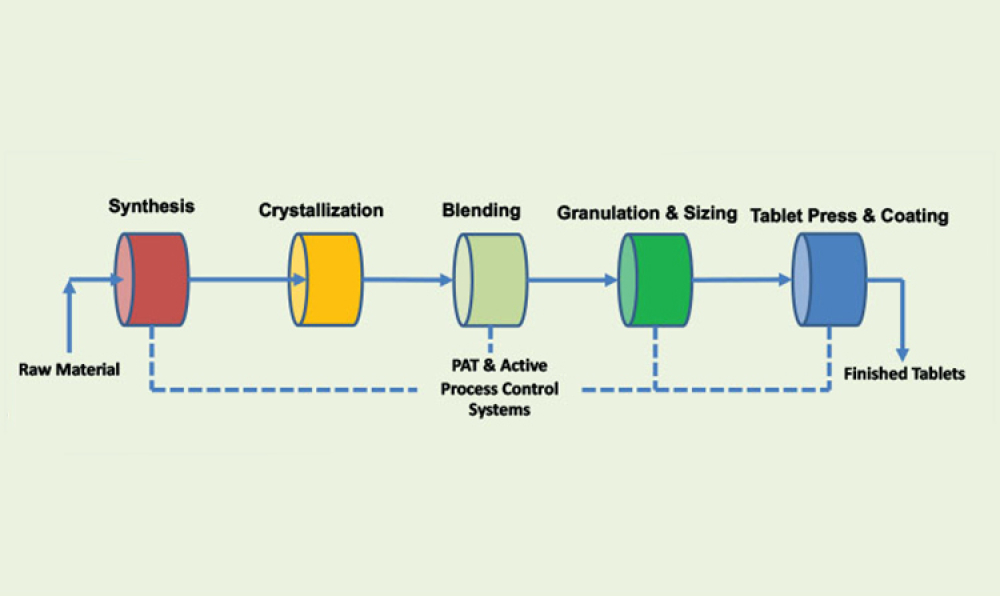
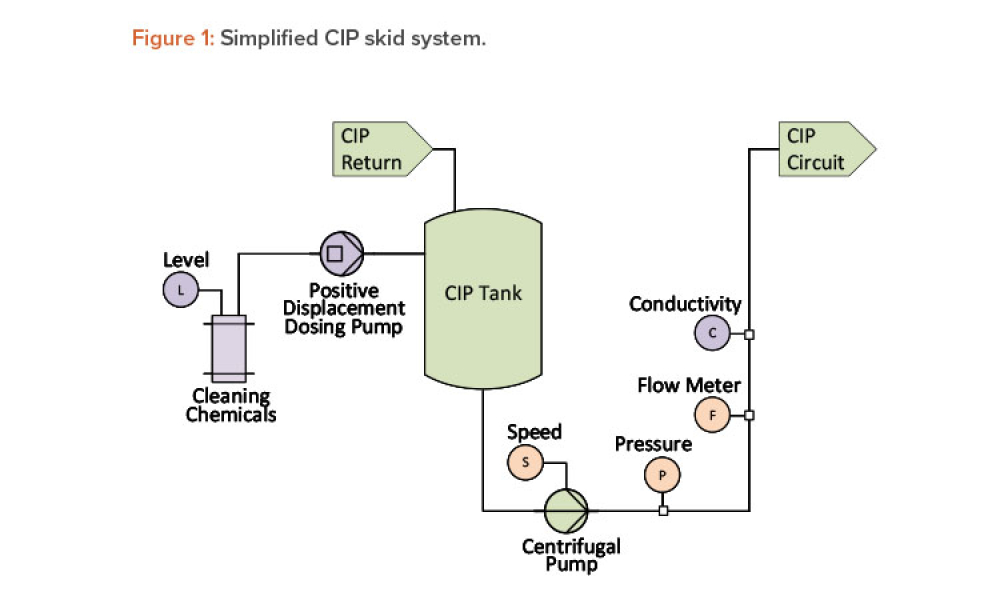
Process Analytical Technology (PAT) and lifecycle control strategy are umbrella terms covering a range of essential components for other innovative initiatives, such as quality by design, real-time release, and continuous manufacturing.
Guidance Documents
Knowledge Management (1)
+Lifecycle Management (3)
+Pharma 4.0™ (1)
+Process Analytical Technology (4)
+Quality by Design (2)
+Community Discussions
Community Discussions
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 14, 2025
Advanced Manufacturing
Critical Utilities
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
Quality Risk Management (QRM) Workshop Training Course
+Implementing Process Analytical Technology Training Course
+GAMP® 5 GxP Process Control Training Course
+White Papers
July / August 2024
The explosive growth of advanced therapy medicinal products (ATMPs), particularly cellular…
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…