Bristol-Myers Squibb is the 2020 Facility of the Year Award Winner for Project Execution for their Multi-Purpose Cell Culture...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Janssen Pharmaceuticals is the 2020 Facility of the Year Award Winner for Process Innovation for their Mirror 1: A Continuous Manufacturing Platform in Beerse, Belgium.
Eli Lilly and Company is the 2020 FOYA Category Winner for Operational Excellence for their Lilly Innovation Development Center in Indianapolis, Indiana.
Sanofi is the 2020 Facility of the Year Award Winner for Facility of the Future for their Sanofi Digitally Enabled Integrated Continuous Biomanufacturing Facility in Framingham,...
Pfizer, Inc. is the 2020 Facility of the Year Award Winner for Facility Integration for their Andover Clinical Manufacturing Facility (ACMF) in Andover, Massachusetts.
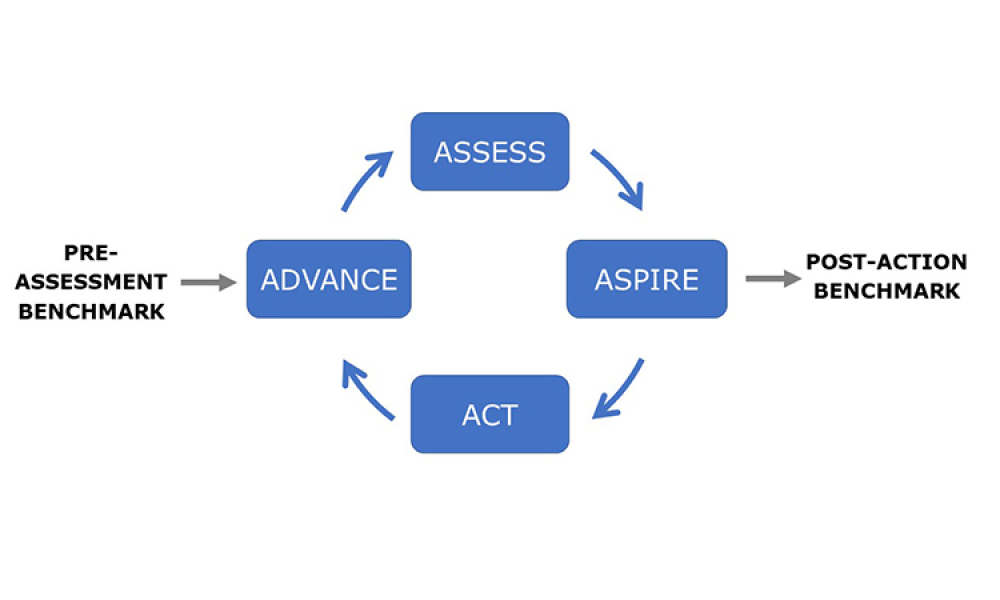
Process validation (PV) aims at reassuring a manufacturer of constant product quality. Moreover, it is a regulatory requirement to achieve licensure of a pharmaceutical product Failing to efficiently plan and execute activities here leads to increased time-to-market. Statistics play a pivotal role here, as is shown by the fact that in the 22 pages of the latest FDA guidance document on process...
The needs of patients must always be at the forefront of what we do.