Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Wow! As a Founding Member of ISPE, you’ve been with the Society for 40 years! Can you provide a brief summary of your involvement with ISPE and share some of the more interesting experiences and successes you’ve had with the Society during your lengthy tenure?
2020 ISPE Biopharmaceutical Manufacturing Virtual Conference
ISPE’s first virtual conference, the 2020 ISPE Biopharmaceutical Manufacturing Virtual Conference, is a new...
Our Young Professionals (YPs) community has been growing over the last couple of years and we are working hard building Young Professionals groups within European Affiliates. In this edition of ISPE Update, we would like...
As ISPE celebrates the 40th anniversary of its establishment, we would like to recognize those members that have been with ISPE throughout its journey. Let’s begin with James Agalloco, who joined ISPE in October 1980. We talked with James to learn about his many years with ISPE, his experiences and accomplishments, and his thoughts on ISPE as we move forward in the future.
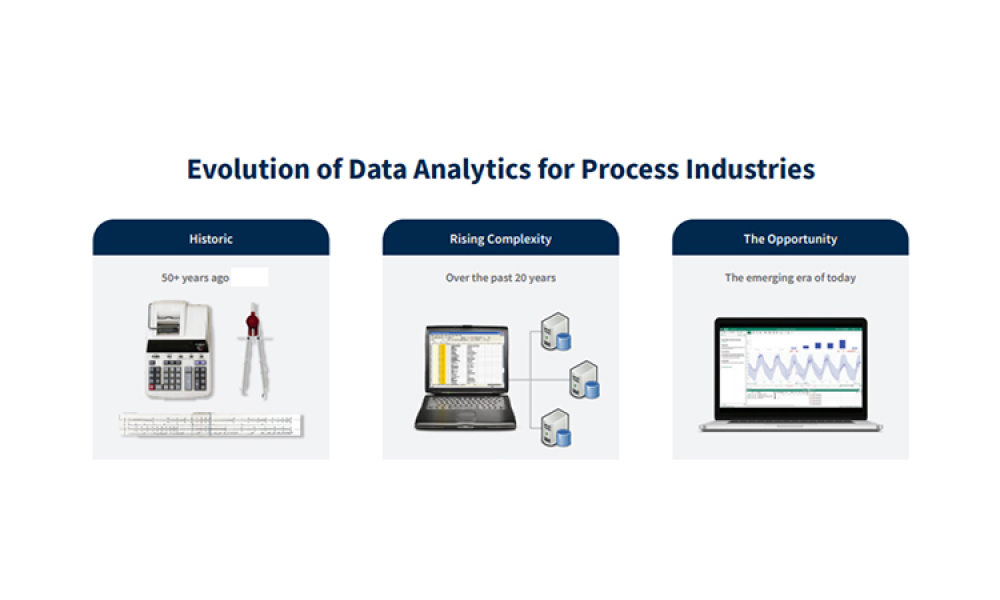
Example case studies show how the evolution of advanced analytics enables innovation in pharma.
Transfer of manufacturing processes and analytical procedures between facilities or laboratories is a necessary part of pharmaceutical development and commercialization. Technology transfers take the outputs of process or method development activities and transfers the knowledge to a different location where a process or analytical procedure will be operated.