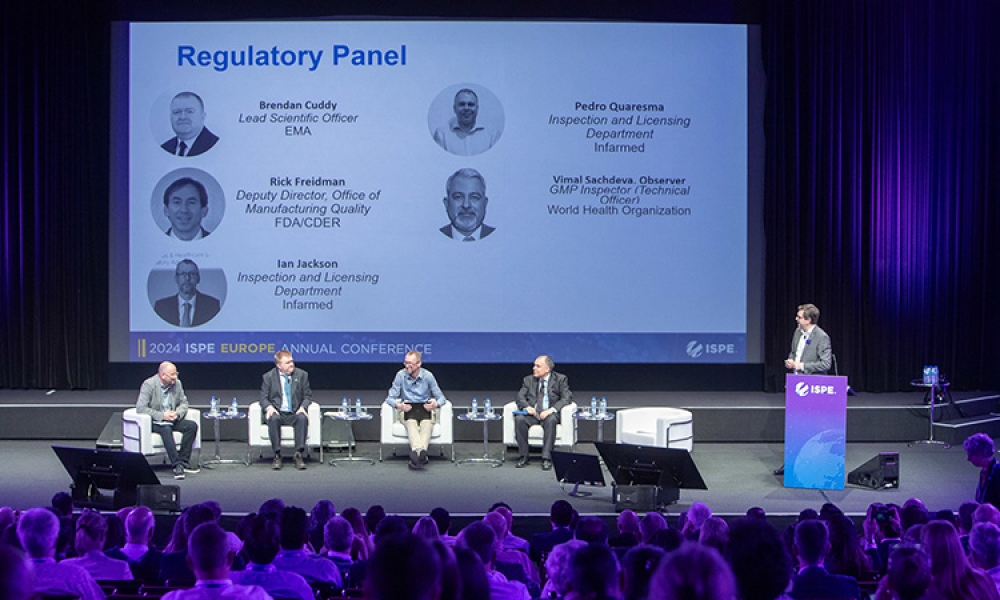
This blog summarizes a presentation by Rick Friedman, Deputy Director of the US FDA Office of Manufacturing Quality, on 17 April 2024 at the 2024 ISPE Europe Annual Conference in Lisbon, Portugal. The...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
One of the keynote sessions of the 2024 ISPE Europe Annual Conference held 16-18 April in Lisbon, Portugal was a presentation on EU initiatives to advance digitalization in manufacturing by Evdokia...
ISPE connected with Sydney Hamilton, Strategic Facility Planner, CRB, and Scott McNallan, Director of Architectural Design, CRB, for a sneak peek look at their planned session, “Blank Slate to Building Excellence,” ahead of the 2024 ISPE Biotechnology Conference. The conference will take place 17-18 June 2024 in Boston, Massachusetts, USA.
Rise of the Machines
It becomes clear that a topic has become mainstream when it crosses multiple Communities of Practice and areas of interest. In preparation for the 2024 ISPE Annual Meeting & Expo, the Digital Transformation track committee worked closely with multiple teams from other tracks on the topic of artificial intelligence/machine learning (AI/ML) to identify the best...
Green chemistry is a field of chemistry that focuses on developing and utilizing more sustainable and environmentally friendly chemical processes and products. Green chemistry is a growing field in pharmaceutical laboratories that aims to reduce the environmental impact of drug discovery, development, and manufacturing processes. It involves the application of sustainable and innovative...
Time has a remarkable ability to slip through our fingers, doesn't it? With June's arrival, we're reminded of how swiftly the year passes. As we transition into the third quarter, it's a pivotal moment for us to reassess our goals and lay the groundwork for the months ahead, even looking ahead to the following year. View the full blog for recent membership announcements, etc.
We've had the opportunity to interview Hilal Yamaner, an active ISPE Emerging Leader and member of ISPE Women in Pharma®. Throughout this member spotlight, she shares her journey with ISPE, her perspective and passion for AI integration, the work she’s put in to planning a related ISPE Women in Pharma panel session at the upcoming
A recently released guidance document, the ISPE Guide: 503A Compounding – Regulatory Basis and Industry Good Practices for Pharmacies, summarizes and contextualizes key information from US governmental bodies relevant to...
On 17 April 2024 at the 2024 ISPE Europe Annual Conference in Lisbon, Portugal, a panel discussion titled “Harmonization to Break Down Barriers to Robust Supply Chains” featured an international panel of experts who discussed drug recalls, drug shortages, risks that can lead to poor drug product quality, quality risk management, Annex 1 implementation and its effect on the supply chain,...
Eli Lilly has recently achieved a remarkable milestone with the completion of its new synthetic peptide manufacturing facility/platform at its facility in Kinsale, Ireland. This cutting-edge project not only enhances production capabilities but also exemplifies a commitment to innovation and safety. Below is an overview which delves into the details of this groundbreaking achievement and...
ISPE and the ISPE Foundation mourn the loss of a beloved member, mentor, and leader.
Building off the success of the 2023 ISPE Annual Meeting & Expo, the program committee has developed a robust workshop program, taking place Sunday, 13 October, which is based on the practical application of the ISPE Baseline Guide and ISPE Good Practice Guide (GPG) Series, covering the following key areas:
Novel therapies refer to innovative and often groundbreaking approaches to treating medical conditions. These therapies typically involve new modalities aiming to improve upon existing treatments or to provide entirely new options for patients. The development of novel therapies is not immune to the challenges of standard therapeutic pursuits; however, the use of automation can significantly...
In the bustling city of Boston, amidst the vibrant biotech community, the 2024 ISPE Biotechnology Conference will gather industry leaders and innovators for a pivotal set of discussions on Track 4: Lifecycle...
The ISPE Advancing Pharmaceutical Quality (APQ) program team has been actively engaged with the US Food and Drug Administration’s (US FDA) Quality Management Maturity (QMM) program since its inception and along the...
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) has recently announced the adoption of the revised guideline
The outlook for the biopharmaceutical market is promising, with expectations that the market will double in the next 10 years, resulting in new therapies and advances in biopharmaceutical manufacturing. This doesn't sound like much at first, but if you consider how the market has developed over the last 20 years, a doubling in the next 10 years is very significant.
The following blog post was provided by Peyton Myers, an undergraduate student at Appalachian State University. Myers attended the 2023 ISPE Annual Meeting & Expo in Las Vegas as an ISPE Foundation Professional Development Grant recipient.