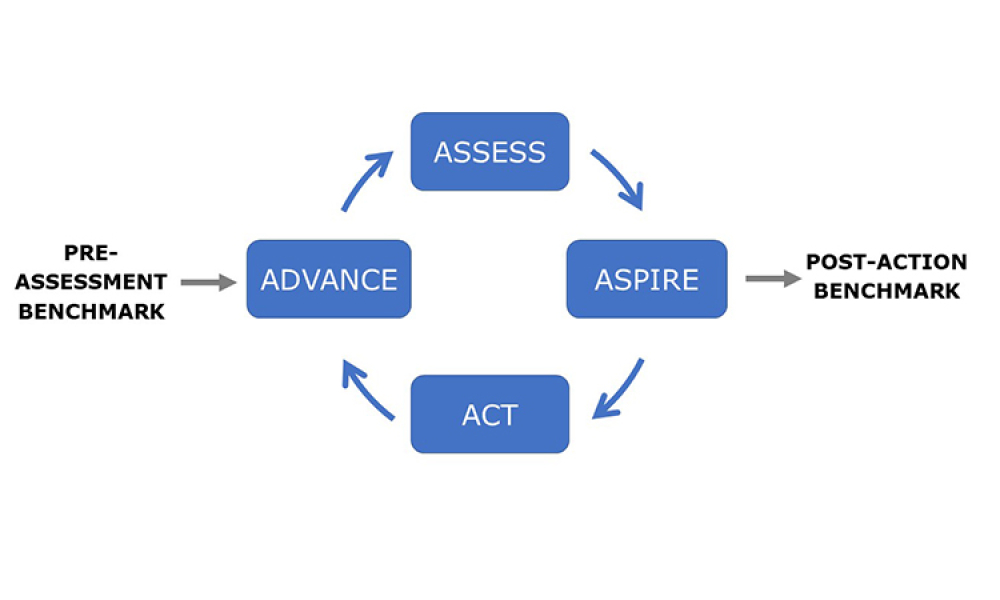
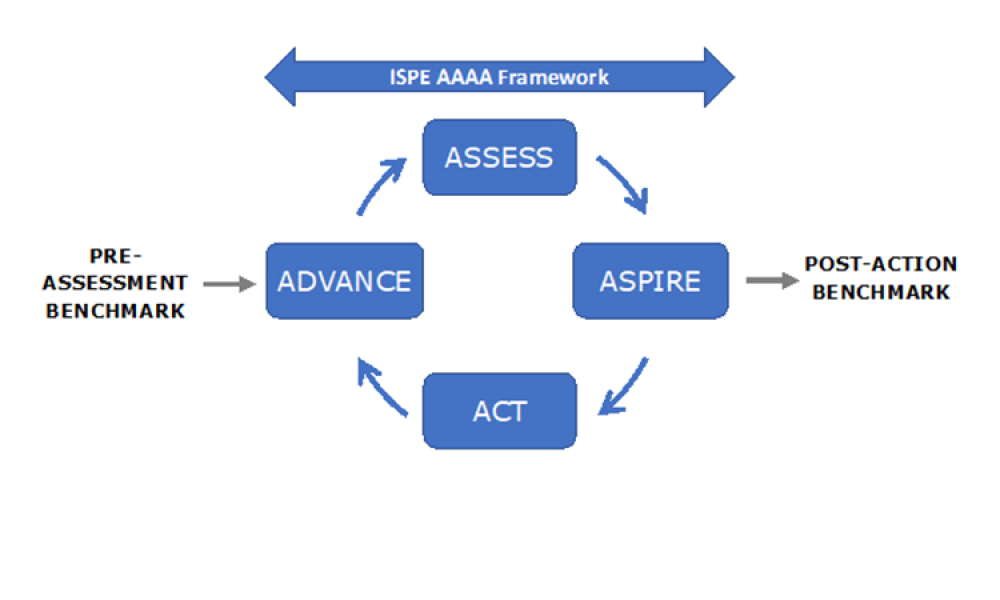
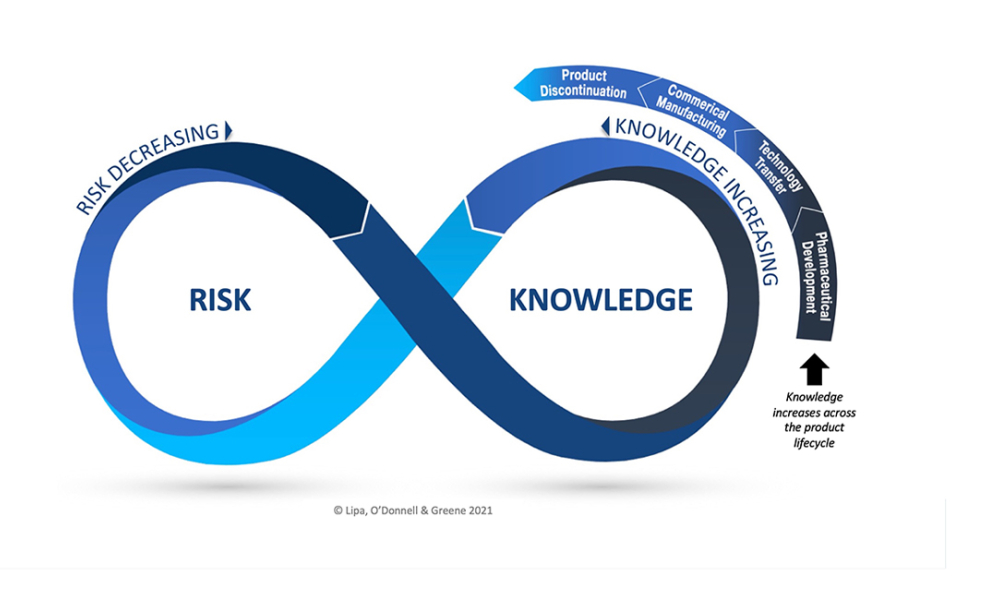
Knowledge management efficiently handles information and resources within a commercial organization. The purpose of knowledge management is to share perspectives, ideas, experiences, and information; to ensure the information is available in the right place at the right time to enable informed decisions and to improve efficiency by reducing the need to rediscover knowledge. Knowledge management consists of three areas: accumulating, storing, and sharing knowledge.
Guidance Documents
Data Integrity (1)
+GAMP® (1)
+Knowledge Management (7)
+Lifecycle Management (1)
+Process Analytical Technology (1)
+Quality Assurance (4)
+Community Discussions
Community Discussions
Apr 08, 2025
Validation
Apr 08, 2025
Data Integrity
Apr 07, 2025
Advanced Manufacturing
Biotechnology
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Mar 20, 2025
Mar 20, 2025
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
ICH Q9(R1): Guidelines on Pharmaceutical Risk Management
+ICH Q6A: Specifications, Test Procedures, and Acceptance Criteria for New Drug Substances and Products
+GxPs for Leadership
+GMP Refresher
+Advancing Pharmaceutical Quality (APQ) Quality Management Maturity Training Course
+White Papers
July / August 2024
The explosive growth of advanced therapy medicinal products (ATMPs), particularly cellular…
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…