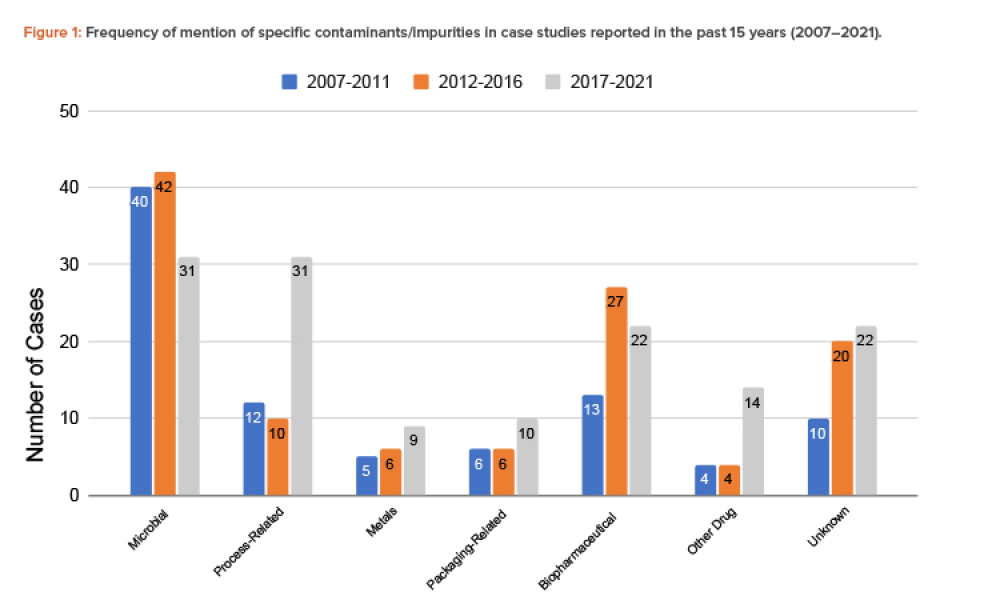
Microbiological and viral contamination control (Microbio Contam Ctrl) refers to the non-intended or accidental introduction of infectious material like bacteria, yeast, mold, fungi, virus, prions, protozoa, or their toxins and by-products.
Guidance Documents
Containment (3)
+Critical Utilities (6)
+Manufacturing Operations (2)
+Microbiological & Viral Contamination Control (17)
+Oral Solid Dosage (1)
+Quality Control (2)
+Quality by Design (1)
+Regulatory (2)
+Sterile Products (1)
+Sustainability (1)
+Sustainable Facilities, HVAC, & Controlled Environments (4)
+Validation (1)
+Community Discussions
Community Discussions
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Apr 08, 2025
Validation
Apr 08, 2025
Data Integrity
Apr 07, 2025
Advanced Manufacturing
Biotechnology
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Mar 20, 2025
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
Aseptic Processing & Annex 1 Training Course
+HVAC & Environmental Control for Life Science Facilities Training Course
+Cleaning Validation Principles Training Course
+CIP System Design, Integration and CIP Chemicals Training Course
+Featured Conferences

White Papers
July / August 2024
The explosive growth of advanced therapy medicinal products (ATMPs), particularly cellular…
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…









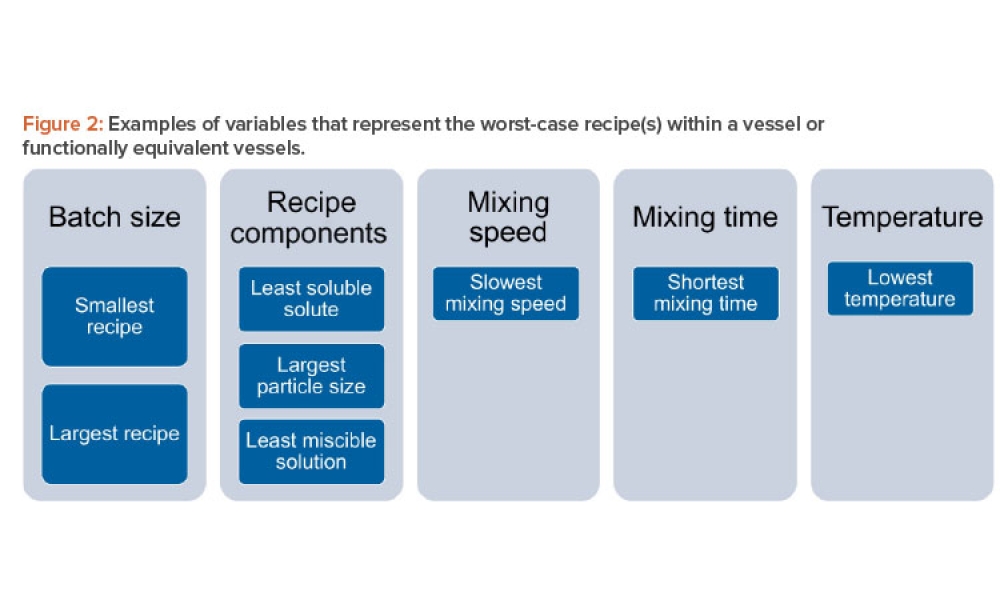





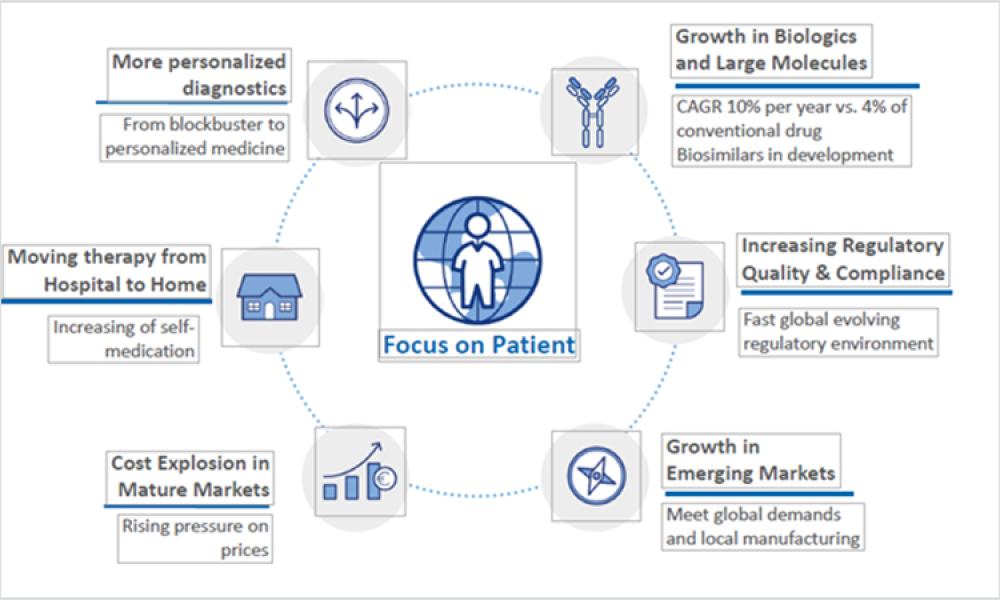

![Figure 1: Seasonal comfort (temperature and RH). ©ASHRAE, www.ashrae.org. Used with permission from 2017 ANSI/ASHRAE Standard 55 [4].](/sites/default/files/styles/teaser_image/public/2021-08/0921_PE_SO_TechHaycock_01.jpg?itok=mJiPGN_X)